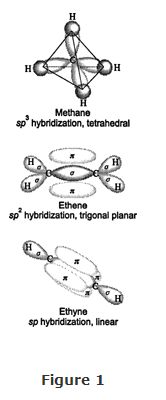
Three‐Dimensional Shapes of Molecules
The overall shape of an organic molecule is fixed by the shape of the central carbon atoms, which compose the backbone of the molecule. The shape of this backbone is determined by the types of hybrid orbitals making up the bonds between the central carbon atoms. If the central carbon atoms are sp 3 hybridized, the molecule will possess a tetrahedral shape. Central carbon atoms that are sp 2 hybridized lead to trigonal‐planar shapes, while sp hybridization produces linear molecules. Three‐dimensional representations of methane ( sp 3 hybridization), ethene ( sp 1 hybridization), and ethyne ( sp hybridization) molecules are shown in Figure .