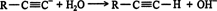Alkynes of the general structure

![]()
are referred to as terminal alkynes. These types of alkynes are weakly acidic. Exposure to a strong base, such as sodium amide, produces an acid‐base reaction.
![]()
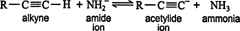
![]()
The acidity of a terminal alkyne is due to the high level of s character in the sp hybrid orbital, which bonds with the s orbital of the hydrogen atom to form a single covalent bond. The high level of s character in an sp‐hybridized carbon causes the overlap region of the σ bond to shift much closer to the carbon atom. This polarizes the bond, causing the hydrogen atom to become slightly positive. This slight positive charge makes the hydrogen atom a weak proton, which can be removed by a strong base.
In the case of alkanes and alkenes, the s character in the hybridized carbon bonds is less, resulting in fewer electronegative carbon atoms and a corresponding lesser shift toward those atoms in the overlap region of the σ bond. The location of the overlap region makes the corresponding hydrogen atoms less electron deficient and thus less acidic. In reality, the hydrogen atoms bonded to alkanes and alkenes can be removed as protons, but much stronger nonaqueous bases are necessary.
The reaction that forms the acetylide ion is reversible. Thus, the base may not form an acid of greater strength than the starting alkyne by acceptance of the proton, or the newly formed conjugate acid will reprotonate the acetylide ion. The fact that stronger acids are capable of reprotonating the acetylide ion can be seen in its reaction with water.