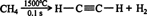The preparations of alkynes are very similar to those of the alkenes. The main preparative reactions involve the elimination of groups or ions from molecules, resulting in the formation of π bonds.
Dehydrohalogenation. The loss of a hydrogen atom and a halogen atom from adjacent alkane carbon atoms leads to the formation of an alkene. The loss of additional hydrogen and halogen atoms from the double‐bonded carbon atoms leads to alkyne formation. The halogen atoms may be located on the same carbon (a geminal dihalide) or on adjacent carbons (a vicinal dihalide). ![]()
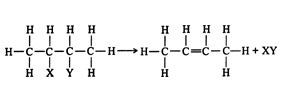
During the second dehydrohalogenation step, certain conditions are necessary, namely high temperatures and an extremely strong basic solution.
Dehalogenation. Vicinal tetrahaloalkanes can be dehalogenated with zinc metal in an organometallic reaction to form alkynes. ![]()
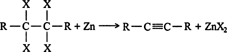
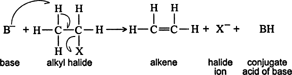
Substitution. Larger alkynes can be generated by reacting an alkyl halide with an acetylide ion, which is generated from a shorter alkyne. ![]()
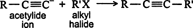
Because acetylide ions are bases, elimination reactions can occur, leading to the formation of an alkene from the alkyl halide. Because substitution and elimination reactions proceed through the formation of a common intermediate, these two types of reactions always occur simultaneously. ![]()
Ethyne (acetylene) preparation. Ethyne, which is commonly called acetylene, is the simplest alkyne. Historically, it was prepared by reacting calcium carbide with water. ![]()
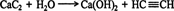
Today, ethyne is normally prepared by the pyrolysis of methane. In this procedure, a stream of methane gas is briefly heated to 1500°C in an airless chamber. Air must be excluded from the reaction or oxidation (combustion) will occur.