Alkynes: Addition Reactions
The principal reaction of the alkynes is addition across the triple bond to form alkanes. These addition reactions are analogous to those of the alkenes.
Hydrogenation. Alkynes undergo catalytic hydrogenation with the same catalysts used in alkene hydrogenation: platinum, palladium, nickel, and rhodium. Hydrogenation proceeds in a stepwise fashion, forming an alkene first, which undergoes further hydrogenation to an alkane. ![]()
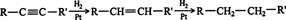
This reaction proceeds so smoothly that it is difficult, if not impossible, to stop the reaction at the alkene stage, although by using palladium or nickel for the catalyst, the reaction can be used to isolate some alkenes. Greater yields of alkenes are possible with the use of poisoned catalysts. One such catalyst, the Lindlar catalyst, is composed of finely divided palladium coated with quinoline and absorbed on calcium carbonate. This treatment makes the palladium less receptive to hydrogen, so fewer hydrogen atoms are available to react. When a catalyst is deactivated in such a manner, it is referred to as being poisoned.
The mechanism of alkyne hydrogenation is identical to that of the alkenes. Because the hydrogen is absorbed on the catalyst surface, it is supplied to the triple bond in a syn manner.
Alkynes can also be hydrogenated with sodium in liquid ammonia at low temperatures. This reaction is a chemical reduction rather than a catalytic reaction, so the hydrogen atoms are not attached to a surface, and they may approach an alkene from different directions, leading to the formation of trans alkenes. ![]()
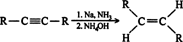
Halogenation. The addition of halogens to an alkyne proceeds in the same manner as halogen addition to alkenes. The halogen atoms add to an alkyne molecule in a stepwise fashion, leading to the formation of the corresponding alkene, which undergoes further reaction to a tetrahaloalkane. ![]()
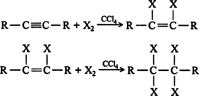
Unlike most hydrogenation reactions, it is possible to stop this reaction at the alkene stage by running it at temperatures slightly below 0°C.
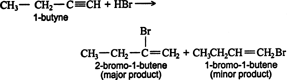
Hydrohalogenation. Hydrogen halides react with alkynes in the same manner as they do with alkenes. ![]()
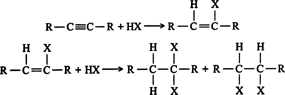
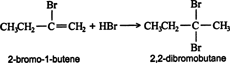
Both steps in the above addition follow the Markovnikov rule. Thus, the addition of hydrogen bromide to 1‐butyne gives 2‐bromo‐1‐butene as the major product of the first step.
The reaction of 2‐bromo‐1‐butene in the second step gives 2,2‐dibromobutane as the major product. ![]()
Hydration. The addition of the elements of water across the triple bond of an alkyne leads to the formation of aldehydes and ketones. Water addition to terminal alkynes leads to the generation of aldehydes, while nonterminal alkynes and water generate ketones.
These products are produced by rearrangement of an unstable enol (vinyl alcohol) intermediate. The term “enol” comes from the en in “alkene” and ol in “alcohol,” reflecting that one of the carbon atoms in vinyl alcohol has both a double bond (alkene) and an OH group (alcohol) attached to it. A vinyl group is
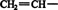
![]()
and a vinyl alcohol is ![]()
![]()
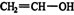
![]()
Water adds across the triple bond of an alkyne via a carbocation mechanism. Dilute mineral acid and mercury(II) ions are needed for the reaction to occur. ![]()
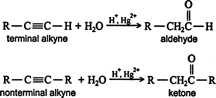
The first step of the mechanism is an acid‐base reaction between the mercury(II) ion (Hg 2+) and the π system of the alkyne to form a π complex. ![]()
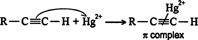
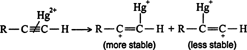
The π complex is converted into a single bond between one or the other of the carbons of the triple bond and the mercury (II) ion, with the resulting generation of a carbocation. ![]()
A molecule of water is attracted to the carbocation to form an oxonium ion. ![]()
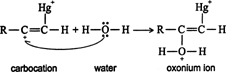
The oxonium ion loses a proton to stabilize itself. ![]()
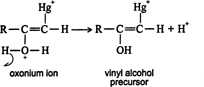
The vinyl alcohol precursor that results is converted into vinyl alcohol (enol) by reaction with a hydronium ion (H 3O +). ![]()
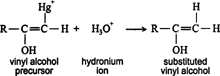
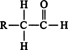
Vinyl alcohols (enols) are unstable intermediates, and they undergo rapid isomerization to form ketones. Such isomerization is called keto‐enol tautomerism. ![]()
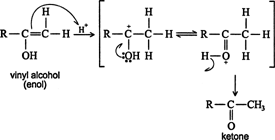
In a similar fashion, the less‐stable intermediate generates an aldehyde. ![]()
Oxidation. Alkynes are oxidized by the same reagents that oxidize alkenes. Disubstituted alkynes react with potassium permanganate to yield vicinal diketones (Vic‐diketones or 1,2‐diketones) or, under more vigorous conditions, carboxylic acids. ![]()
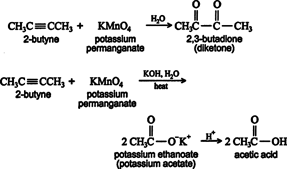
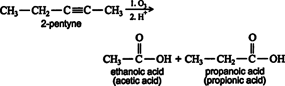
Ozonolysis of an alkyne also leads to carboxylic acid formation. ![]()
Polymerization. Alkynes can be polymerized by both cationic and free‐radical methods. The reactions and mechanisms are identical with those of the alkenes.