Polymerization is a process by which an organic compound reacts with itself to form a high‐molecular‐weight compound composed of repeating units of the original compound. The polymerization of ethene by an ionic, or free‐radical, reagent A−B is an example.
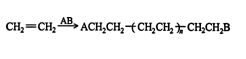
Polymerization reactions proceed via either cationic or free‐radical mechanisms. In both processes, π bonds are converted to σ bonds, and energy is liberated. Cationic polymerization is less efficient than free‐radical polymerization due to the caustic nature of cation‐producing reagents. An example of a cation‐initiated polymerization is the reaction of ethene with sulfuric acid. ![]()
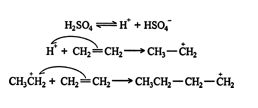
The reaction continues and gives
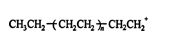
which finally reacts with HSO 4 − to create the polymer ![]()
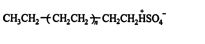
![]()
The more effective free‐radical polymerization can be initiated by oxygen or other free‐radical compounds, such as peroxides. The free‐radical polymerization of ethene by an alkoxide radical is a typical reaction. ![]()
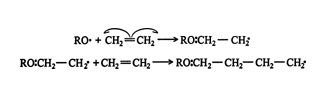
The reaction continues and gives ![]()
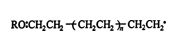
The reaction may end by one of two termination steps. One is the bonding of two free radicals
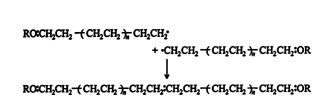
and the other is the internal stabilization of the polymer by double‐bond formation.
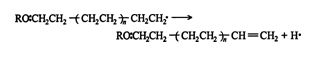
![]()
![]()