The positively charged end carbon of the carbocation attracts the electrons in the overlap region that bond it to the adjacent a carbon. This electron movement makes the α carbon slightly positive, which in turn attracts the electrons in the overlap regions of all other atoms bonded to it. This results in the hydrogen on the α carbon becoming very slightly acidic and capable of being removed as a proton in an acid‐base reaction.
Zaitsev rule. It may be possible in some instances to create a double bond through an alcohol dehydration reaction in which hydrogen atoms are lost from two different carbons on the carbocation. The major product is always the more highly substituted alkene, that is, the alkene with the greater number of substituents on the carbon atoms of the double bond, an observation called the Zaitsev rule. Thus, in the dehydration reaction of 2‐butanol, the following products are formed. ![]()
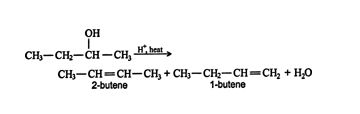
The Zaitsev rule predicts that the major product is 2‐butene. Notice that each carbon atom involved in the double bond of 2‐butene has one methyl group attached to it. In the case of 1‐butene, one carbon atom of the double bond has one substituent (the ethyl group), while the other carbon atom has no substituents.
Carbocation rearrangement. The carbocation in an alcohol dehydration may undergo rearrangement to form more stable arrangements. Dehydration of 2‐methyl‐3‐pentanol, for example, leads to the production of three alkenes. The mechanism for the reaction shows that the extra compound formation is due to rearrangement of the carbocation intermediate. ![]()
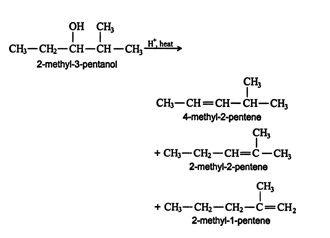
The 2‐methyl‐1‐pentene molecule is formed via rearrangement of the intermediate carbocation. ![]()
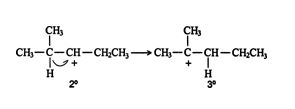
The movement of a hydride ion (H: ‐) leads to the formation of a more stable carbocation. Carbocations are classified as primary, secondary, and tertiary, as are the carbon atoms. A primary carbocation has one alkyl group attached to it; a secondary carbocation is bonded to two alkyl groups; and a tertiary carbocation has three alkyl groups around it. ![]()
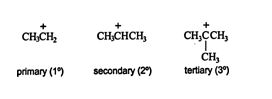
Alkyl groups theoretically have the ability to “push” electrons away from themselves. This phenomenon is called the inductive effect. The greater the number of alkyl groups “pushing” electrons toward a positively charged carbon atom, the more stable the intermediate carbocation will be. This increase in stability is due to the delocalization of charge density. A charge on an atom creates a stress on that atom. The more the stress is spread over the molecule, the smaller the charge density becomes on any one atom, reducing the stress. This lessening of stress makes the ion more stable. Thus, tertiary carbocations, with three alkyl groups on which to delocalize the positive charge, are more stable than secondary carbocations, which have only two alkyl groups on which to delocalize the positive charge. For the same reason, secondary carbocations are more stable than primary carbocations.
In reality, alkyl groups do not “push” electrons away from themselves, but rather they have electrons removed from them. When an atom picks up a positive charge and becomes an ion, its electronegativity changes. In the original σ bond between two carbon atoms, the location of the overlap region relative to each carbon atom is fixed in part by the electronegativity of the two atoms. With an increase in the electronegativity of one of the carbon atoms due to ion formation, the overlap region shifts closer to the more electronegative, positively charged carbon atom. This rearrangement of electron density produces a partial positive charge on the neighboring carbon. The amount of charge gained by the second carbon corresponds to the amount lost by the fully charged carbon atom. In this manner, the charge becomes delocalized over the two carbons.
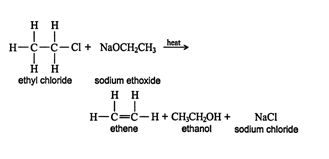
Dehydrohalogenation of alkyl halides. The dehydrohalogenation of alkyl halides, another β elimination reaction, involves the loss of a hydrogen and a halide from an alkyl halide (RX). Dehydrohalogenation is normally accomplished by reacting the alkyl halide with a strong base, such as sodium ethoxide. ![]()
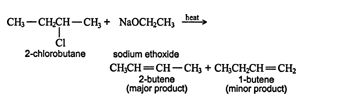
This reaction also follows the Zaitsev rule, so in the reaction of 2‐chlorobutane with sodium ethoxide, the major product is 2‐butene. ![]()
Dehydrohalogenation reactions proceed via the following mechanism.
1. A strong base removes a slightly acidic hydrogen proton from the alkyl halide via an acid‐base reaction.
2. The electrons from the broken hydrogen‐carbon bond are attracted toward the slightly positive carbon atom attached to the chlorine atom. As these electrons approach the second carbon, the halogen atom breaks free, leading to the formation of the double bond. The diagram below summarizes this mechanism. ![]()