Alkanes: Molecular and Structural Formulas
The alkanes comprise a series of compounds that are composed of carbon and hydrogen atoms with single covalent bonds. This group of compounds comprises a homologous series with a general molecular formula of C n H 2 n+2 , where equals any integer.
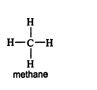
The simplest alkane, methane, has one carbon atom and a molecular formula of CH 4. Because this compound contains only single covalent bonds, its structural formula is ![]()
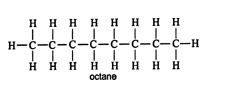
In longer alkane molecules, the additional carbon atoms are attached to each other by single covalent bonds. Each carbon atom is also attached to sufficient hydrogen atoms to produce a total of four single covalent bonds about itself. Thus octane, an eight‐carbon alkane, has a molecular formula of C 8H 18 and a structural formula of ![]()
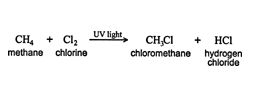
Alkyl groups. When a substituent, such as a halogen or hydroxy group, bonds to an alkane molecule, one of the carbon‐hydrogen bonds of the molecule is converted to a carbon‐substituent bond. For example, when methane reacts with chlorine, a new compound called chloromethane (or methyl chloride) is formed. This new compound contains a CH 3 group bonded to a chlorine atom. ![]()
An alkane with a hydrogen removed from one bond is called an alkyl group. Alkyl groups are often represented by the letter R, just as halogens are often represented by the letter X. The methane‐chlorine reaction can be generalized as ![]()
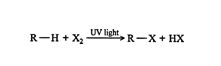
Often, organic chemists use these types of representations for discussing generalized reactions.
Isomers. Alkyl groups can exist in more than one isomeric form. For example, the alkane propane has two alkyl isomers. ![]()
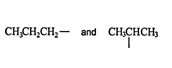
These isomers are distinguished from each other by the type of carbon that has lost a hydrogen to form the alkyl group. Carbon atoms are classified as primary (1°), secondary (2°), or tertiary (3°), depending on the number of carbon atoms to which they are attached. A primary carbon is directly attached to only one other carbon atom. Secondary and tertiary carbons are attached to two and three other carbon atoms, respectively.
In the propane isomer diagram above, the group on the left is a primary (1°) propyl group, while the group on the right is a secondary (2°) propyl group. Butyls (alkanes with four carbons) have three isomeric groups. The structures and names of these groups are
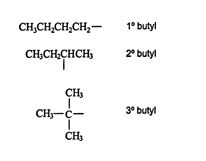
![]()
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|