Alkanes can be oxidized to carbon dioxide and water via a free‐radical mechanism. The energy released when an alkane is completely oxidized is called the heat of combustion. For example, when propane is oxidized, the heat of combustion is 688 kilocalories per mole.
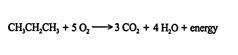
In a homologous series like the straight‐chain alkanes, the energy liberated during oxidation increases by approximately 157 kilocalories for each additional methylene (CH 2) unit.
Heat of combustion data is often used to assess the relative stability of isomeric hydrocarbons. Because the heat of combustion of a compound is the same as the enthalpy of that compound in its standard state, and because potential energy is comparable to enthalpy, the differences in heats of combustion between two alkanes translate directly to differences in their potential energies. The lower the potential energy of a compound, the more stable it is. In the alkanes, the more highly branched isomers are usually more stable than those that are less branched.