Alkenes: Molecular and Structural Formulas
The alkenes comprise a series of compounds that are composed of carbon and hydrogen atoms with at least one double bond in the carbon chain. This group of compounds comprises a homologous series with a general molecular formula of C n H 2 n , where n equals any integer greater than one.
The simplest alkene, ethene, has two carbon atoms and a molecular formula of C 2H 4. The structural formula for ethene is ![]()
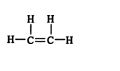
In longer alkene chains, the additional carbon atoms are attached to each other by single covalent bonds. Each carbon atom is also attached to sufficient hydrogen atoms to produce a total of four single covalent bonds about itself. In chains with four or more carbon atoms, the double bond can be located in different positions, leading to the formation of structural isomers. For example, the alkene of molecular formula C 4H 8 has two isomers. ![]()

Stereoisomers. In addition to structural isomers, alkenes also form stereoisomers. Because rotation around a multiple bond is restricted, groups attached to the double‐bonded carbon atoms always remain in the same relative positions. These “locked” positions allow chemists to identify various isomers from the substituents' locations. For example, one structural isomer of C 5H 10 has the following stereoisomers. ![]()
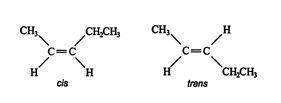
The isomer on the left, in which the two substituents (the methyl and ethyl groups) are on the same side of the double bond, is called the cis isomer, while the isomer on the right, with two nonhydrogen substituents on opposite sides of the double bond, is called the trans isomer.
If more than two substituents are attached to the carbon atoms of a double bond, the cis and trans system cannot be used. With such chemicals, E‐Z notation is used. In the E‐Z system, the molecule is first bisected vertically through the double bond. Second, the two atoms or groups on each carbon atom are ranked by atomic weight. The higher atomic weight is assigned priority. For example, in Figure , the carbon and chlorine atoms on the left side of the bisecting line are ranked. Chlorine has priority because it is heavier. On the right side, bromine outranks carbon. Third, the positions of the two atoms of higher rank are determined. If the two atoms are in the cis position, the arrangement is Z (for German zusammen, meaning “together”). If the atoms or groups are in the trans position, the arrangement is E (for German entgegen, meaning “opposite”).
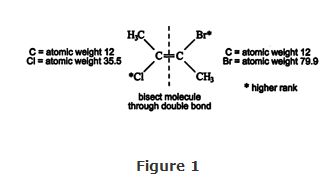
The name of the chemical in Figure is ( E)‐2‐bromo‐3‐chloro‐2‐butene.