Stereochemistry of Reactions
Many simple reactions involve stereochemistry.
A reaction that involves only achiral reactants and reagents can produce only racemic mixtures or products that are achiral. Thus, the monobromination of butane produces a mixture of 1‐bromobutane and 2‐bromobutane. The 1‐bromobutane has no stereogenic center and is, therefore, achiral. The 2‐bromobutane, which has a stereogenic center, is a mixture of the enantiomers ( R)‐2‐bromobutane and ( S)‐2‐bromobutane. This racemic mixture results because the reaction proceeds via a free‐radical mechanism. Free radicals are almost perfectly planar. This planarity makes the product mixture achiral because there is equal probability of a bromine free radical attacking from either side of the carbon free radical intermediate.
Addition of a halogen atom to an alkene proceeds via a trans addition. This occurs due to steric hindrance created by the formation of the halonium ion.
Catalytic hydrogenation of alkenes and alkynes takes place via cis addition because absorption of the hydrogen on the surface of the rigid catalyst allows the hydrogen atoms to approach the double bond from only one side of the alkene molecule.
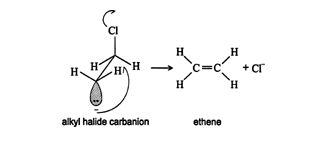
Dehydrohalogenation of chloroethane to form ethene proceeds via a trans elimination reaction because of the tetrahedral shape of the carbon atom bonded to the chlorine atom. To displace the chlorine as a chloride ion, the electron pair of the other carbon must approach from the unhindered side opposite the chlorine atom.
![]()
Notice the use of the sawhorse projection to show how this reaction occurs.