Bond Rupture and Formation
Chemical reactions involve bond rupture and formation. In covalently bonded carbon molecules, for example, the bonds can be broken in two ways: symmetrically or asymmetrically. In a symmetrical rupture, each atom in the original covalent bond receives one electron. This type of rupture generates free radicals and is referred to as homolytic cleavage. In reactions, it generates free‐radical mechanisms.
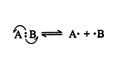
Asymmetrical breaking of a single covalent bond leads to ion formation and is referred to as heterolytic cleavage. In reactions, it generates carbocation or carbanion mechanisms. (A carbocation is a carbon atom bearing a positive charge; a carbanion is a carbon atom bearing a negative charge.) ![]()
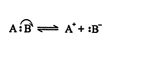
The reverse reactions produce either homogenic bond formation from free radicals or heterogenic bond formation from ions.
|
|
|
|
|
|
|
|