Brønsted‐Lowry Theory of Acids and Bases
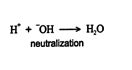
In the early twentieth century, S. Arrhenius defined an acid as a compound that liberates hydrogen ions and a base as a compound that liberates hydroxide ions. In his acid‐base theory, a neutralization is the reaction of a hydrogen ion with a hydroxide ion to form water.
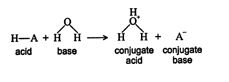
The weakness of Arrhenius's theory is that it is limited to aqueous systems. A more general acid‐base theory was devised by Brønsted and Lowry a couple decades later. In their theory, an acid is any compound that can donate a proton (hydrogen ion). A base is similarly defined as any substance that can accept a proton. This definition broadened the category of bases. In a Brønsted‐Lowry neutralization, an acid donates a proton to a base. In the process, the original acidic molecule becomes a conjugate base; that is, it can accept a proton. Likewise, the base that accepted the proton becomes a conjugate acid, and it can donate a proton. Thus, in a Brønsted‐Lowry neutralization reaction, conjugate acid‐base pairs are generated. ![]()
The ability of a compound to liberate protons is a measure of its strength as an acid. For a compound to easily liberate a proton, its conjugate base must be weak. Similarly, a substance that liberates protons poorly must have a conjugate base that is strong. Thus, the conjugate bases of strong mineral acids are weak, while the conjugate bases of weak inorganic and organic acids are strong.
|
|
|
|
|
|
|
|