Racemic Mixtures: Resolving Enantiomers
Enantiomorphic pairs show no rotation of plane‐polarized light if they are in a true 1:1 mixture. Again, such mixtures are referred to as racemic mixtures, or racemates.
Racemic mixtures can be separated, or resolved, into their pure enantiomers by three methods. The first method is to mechanically separate the crystals in such a mixture based on differences in their shapes. This was the method first used by Pasteur, and it is mainly of historical interest.
The second resolution method employs enzymes. Enzymes are stereospecific chiral protein molecules that act as catalysts. Because of their chirality, these molecules react with only one enantiomer in a racemic mixture. The enantiomer that momentarily bonds to an enzyme undergoes reaction, while the enantiomer that does not bond remains unchanged. The unreacted enantiomer can then be removed from the reaction mix by ordinary separation methods, such as distillation or recrystallization.
The third method involves converting the enantiomers of a racemic mixture into diastereomers and then resolving that mixture with ordinary separation techniques. The separated diastereomers are then treated with appropriate reagents to regenerate the original enantiomers.
![]()
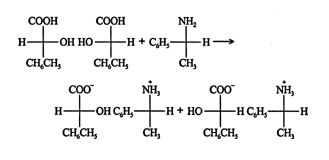
In this example, the diastereomer salts are separated by recrystallization, and the original acids are regenerated by the addition of a hydrochloric acid solution.