Alkenes: Hydrohalogenation
Unlike halogens, hydrogen halides are polarized molecules, which easily form ions. Hydrogen halides also add to alkenes by electrophilic addition.
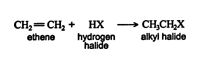
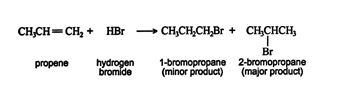
The addition of hydrogen halides to asymmetrically substituted alkenes leads to two products.
![]()
The major product is predicted by the Markovnikov rule, which states that when a hydrogen halide is added to an asymmetrically substituted alkene, the major product results from the addition of the hydrogen atom to the double‐bonded carbon that is attached to more hydrogen atoms, while the halide ion adds to the other double‐bonded carbon. This arrangement creates a more stable carbocation intermediate.
Hydrohalogenation mechanisms. The first step in the addition of a hydrogen halide to an alkene is the dissociation of the hydrogen halide.
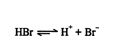
![]()
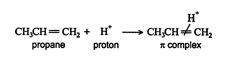
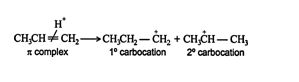
The H + ion is attracted to the π‐bond electrons of the alkene, which forms a π complex.
![]()
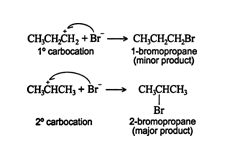
The π complex then breaks, creating a σ single bond between one carbon of the double‐bonded pair and the hydrogen. The carbon atom that loses a share of the π bond then becomes a carbocation. In asymmetrically substituted alkenes, two different carbocations are possible. The major product is generated from the more stable carbocation, while the minor product forms from the less stable one.
![]()
Thus, the major product is 2‐bromopropane.
![]()
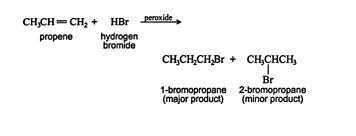
Hydrogen bromide can also be added to an alkene in an anti‐Markovnikov fashion. In anti‐Markovnikov additions, the hydrogen atom of the hydrogen halide adds to the carbon of the double bond that is bonded to fewer hydrogen atoms. For this to result, the reaction must proceed by a noncarbocation intermediate; thus in the presence of peroxide, the reaction proceeds via a free‐radical mechanism, with the major product being generated from the more stable free radical.
![]()
The mechanism for this reaction starts with the generation of a bromine free radical by the reaction of hydrogen bromide with peroxide.
![]()
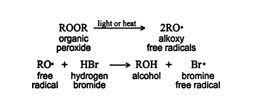
The bromine free radical adds to the alkene, forming a more stable carbon free radical.
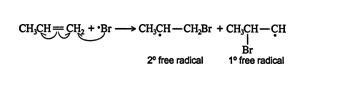
![]()
The secondary free radical is more stable than the primary free radical because the secondary molecule is better able to delocalize the stress placed on the carbon atom by the free‐radical electron. The major product then forms from the intermediates by reacting with hydrogen bromide.
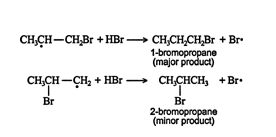
![]()
In all additions of hydrogen halides across carbon‐carbon double bonds, the major product always comes from the more stable intermediate. In Markovnikov additions, the major product results from the more stable carbocation, while in anti‐Markovnikov additions, such as the hydrogen bromide addition in the presence of peroxide, the major product results from the more stable free radical.