Alkenes: Epoxide Reactions
Alkenes are capable of reacting with oxygen in the presence of elemental silver to form a series of cyclic ethers called epoxides. Epoxides are three‐atom cyclic systems in which one of the atoms is oxygen. The simplest epoxide is epoxyethane (ethylene oxide).

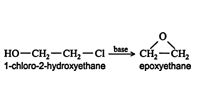
Epoxyethane belongs to a class of chemicals called heterocyclic compounds. These compounds are cyclic structures in which one (or more) of the ring atoms is a hetero atom, that is, an atom of an element other than carbon. In the laboratory, epoxyethane is prepared by reacting 1‐chloro‐2‐hydroxyethane with a base. ![]()
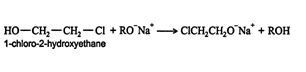
The mechanism for this reaction starts with the base reacting with the acidic hydrogen of the OH group. ![]()
The oxygen anion is then attracted to the carbon that is bonded to the chlorine atom. This carbon bears a strong partial positive charge due to the great differences in electronegativity between the carbon and chlorine atoms. ![]()
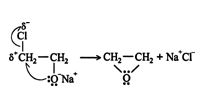
As can be seen in the structural formula above, the oxygen atom must be located anti to the departing chlorine atom for the reaction to occur. The overall reaction is a syn addition.
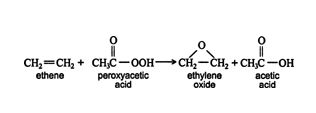
A third method of preparing epoxyethane is by the reaction of an alkene with peroxy acids.