Hybridization of Atomic Orbitals
Physical studies of the simplest organic compound, methane (CH 4), have shown the following:
- all of the carbon‐hydrogen bond lengths are equal
- all of the hydrogen‐carbon‐hydrogen bond angles are equal
- all of the bond angles are approximately 110°
- all of the bonds are covalent
The ground state, or unexcited state, of the carbon atom ( Z = 6) has the following electron configuration.
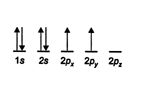
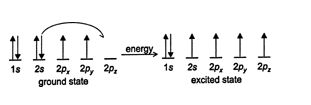
Covalent bonds are formed by the sharing of electrons, so ground‐state carbon cannot bond because it has only two half‐filled orbitals available for bond formation. Adding energy to the system promotes a 2 s electron to a 2 p orbital, with the resulting generation of an excited state. The excited state has four half‐filled orbitals, each capable of forming a covalent bond. However, these bonds would not all be of the same length because atomic 5 orbitals are shorter than atomic p orbitals. ![]()
To achieve equal bond lengths, all the orbitals would have to be the same type. The creation of identical orbitals occurs in nature by a hybridization process. Hybridization is an internal linear combination of atomic orbitals, in which the wave functions of the atomic s and p orbitals are added together to generate new hybrid wave functions. When four atomic orbitals are added together, four hybrid orbitals form. Each of these hybrid orbitals has one part s character and three parts p character and, therefore, are called sp 3 hybrid orbitals.
In the hybridization process, all bond lengths become equal. Bond angles can be explained by the valence‐shell electron‐pair repulsion theory (VSEPR theory). According to this theory, electron pairs repel each other; therefore, the electron pairs that are in bonds or in lone pairs in orbitals around an atom generally separate from each other as much as possible. Thus, for methane, with four single bonds around a single carbon, the maximum angle of repulsion is the tetra‐hedral angle, which is 109°28″, or approximately 110°.
In a similar fashion, the atomic orbitals of carbon can hybridize to form sp 2 hybrid orbitals. In this case, the atomic orbitals that undergo linear combination are one s and two p orbitals. This combination leads to the generation of three equivalent sp 2 hybrid orbitals. The third p orbital remains an unhybridized atomic orbital. Because the three hybrid orbitals lie in one plane, the VSEPR theory predicts that the orbitals are separated by 120° angles. The unhybridized atomic p orbital lies at a 90° angle to the plane. This configuration allows for the maximum separation of all orbitals.
Last, the atomic orbitals of carbon can hybridize by the linear combination of one s and one p orbital. This process forms two equivalent sp hybrid orbitals. The remaining two atomic p orbitals remain unhybridized. Because the two sp hybrid orbitals are in a plane, they must be separated by 180°. The atomic p orbitals exist at right angles to each other, one in the plane of the hybridized orbitals and the other at a right angle to the plane.
The type of hybrid orbital in any given carbon compound can be easily predicted with the hybrid orbital number rule. ![]()
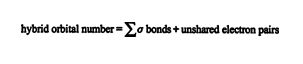
A hybrid orbital number of 2 indicates sp hybridization, a value of 3 indicates sp 2 hybridization, and a value of 4 indicates sp 3 hybridization. For example, in ethene (C 2H 4), the hybrid orbital number for the carbon atoms is 3, indicating sp 2 hybridization. ![]()
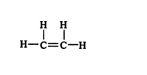
All the carbon‐hydrogen bonds are σ, while one bond in the double bond is σ and the other is π. ![]()

Thus, the carbons have sp 2 hybrid orbitals.
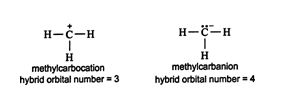
Using the hybrid orbital number rule, it can be seen that the methylcarbocation contains sp 2 hybridization, while the methylcar‐banion is sp 3 hybridized. ![]()