Structural Isomers and Stereoisomers
Isomers are compounds with different physical and chemical properties but the same molecular formula. In organic chemistry, there are many cases of isomerism. For example, the formula C 4H 10 represents both butane and 2‐methylpropane.
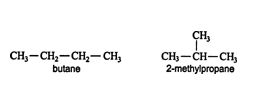
These are examples of structural isomers, or constitutional isomers. Structural isomers have the same molecular formula but a different bonding arrangement among the atoms.
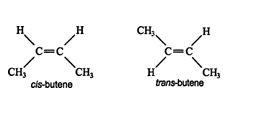
Stereoisomers have identical molecular formulas and arrangements of atoms. They differ from each other only in the spatial orientation of groups in the molecule. The simplest forms of stereoisomers are cis and trans isomers, both of which are created by the restricted rotation about a double bond or ring system. Butene, C 4H 8, exists in both cis and trans forms. ![]()