Although some common alkyne names, such as acetylene, are still found in many textbooks, the International Union of Pure and Applied Chemistry (IUPAC) nomenclature is required for journal articles. The rules for alkynes in this system are identical with those for alkenes, except for the ending. The following rules summarize alkyne nomenclature.
1. Identify the longest continuous chain of carbon atoms that contains the carbon‐carbon triple bond. The parent name of the alkyne comes from the IUPAC name for the alkane of the same number of carbon atoms, except the ‐ ane ending is changed to ‐ yne to signify the presence of a triple bond. Thus, if the longest continuous chain of carbon atoms containing a triple bond has five atoms, the compound is pentyne.
2. Number the carbon atoms of the longest continuous chain, starting at the end closest to the triple bond. Thus, is numbered from right to left, placing the triple bond between the second and third carbon atoms of the chain. (Numbering the chain from left to right incorrectly places the triple bond between the third and fourth carbons of the chain.)
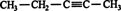
![]()
3. The position of the triple bond is indicated by placing the lower of the pair of numbers assigned to the triple‐bonded carbon atoms in front of the name of the alkyne. Thus the compound shown in rule 2 is 2‐pentyne.
4. The location and name of any substituent atom or group is indicated. For example, the compound is
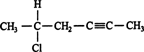
![]()
5‐chloro‐2‐hexyne.
![]()