In many instances, only the valence electrons—also called the outer‐shell electrons or bonding electrons (because of their location in the atom and their reactivity)—are of interest to chemists. In such cases, it is advantageous to draw the Lewis structure of the atom or molecule. In a Lewis structure (also known as an electron dot structure), the entire atom, with the exception of the valence electrons, is represented by the symbol of the element, and the valence electrons are represented by dots. Thus, the Lewis structure of carbon ( Z = 6) is
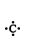
The letter C, the symbol for carbon, represents the carbon nucleus of six protons and six neutrons and the two 1 s electrons. The four outer‐shell electrons, two 2 s and two 2 p electrons, are represented by the dots.
|
|
|
|
|
|
|
|