Stability of Conjugated Systems
Investigations of 1,3‐butadiene have shown that the central single bond is slightly shorter than expected. In addition, the heat of hydrogenation of the molecule, 57.1 kilocalories per mole, is less than the amount predicted from doubling the heat of hydrogenation of two butene molecules (60.6 kcal/mol).
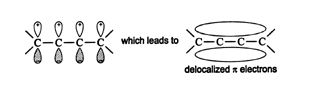
A molecular orbital picture of 1,3‐butadiene shows possible π‐bond overlap throughout the molecule.
![]()
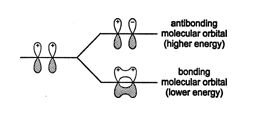
For this type of delocalized bonding to occur, the atomic p orbitals must align, with all the lobes having the same phase signs. Alignment of oppositely signed lobes leads to a higher energy state. ![]()
Thus, a conjugated diene system must look like this. ![]()
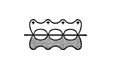
This arrangement produces a low‐energy state. The complete delocalization of the π system gives the single bond some double‐bond character and explains why it is slightly shorter than expected. Rotation around this single bond is also somewhat restricted because of the partial double‐bond character of the bond and because of an increase in repulsion between groups attached to the terminal carbons. The increase in repulsive forces is due to the shorter bond length, which brings the groups closer together.