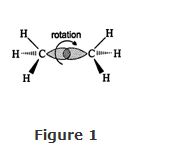
Free Rotation Around Single Bonds
Carbon atoms in single bonds rotate freely. Rotation can occur because the heaviest electron density in the σ bond exists along an imaginary line between two carbon nuclei. Rotation does not change this electron distribution; the bond strength remains constant throughout rotation. Because rotation is possible, the molecule can have an infinite number of conformations, and a sketch of any of them is an accurate representation of the molecule. Some conformations have slightly higher energy content because of repulsion between atoms or between groups bonded to adjacent carbons as they approach each other due to rotation. Rotation around the carbon‐carbon bond in the ethane molecule is shown in Figure .