Cyclohydrocarbons: Reactivity, Stresses of Small Rings
All cycloalkane ring carbon atoms are sp 3 hybridized, requiring bond angles that must be tetrahedral, or approximately 110°. However, three‐ and four‐membered carbon rings are planar, so their bonding angles are 60° and 90°, respectively. The small size of these bond angles compared to the tetrahedral angle means that the orbital overlap region cannot exist directly between two carbon atoms. Rather, the two carbons are located at a slight angle to the overlap region, an arrangement that creates a weaker, more reactive bond. This type of bonding strain is called angle strain. Five‐membered rings have a bond angle of 108°, which is very close to the tetrahedral angle. As a result, this ring system possesses little angle strain. Rings of six carbons or more bend and thus maintain the stable tetrahedral bonding angle.
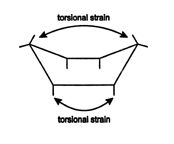
In both the chair and boat forms of cyclohexane, there is no angle strain; however, the boat form has another type of ring strain called torsional strain. Torsional strain is caused by the interaction of hydrogen atoms or substituents that are bonded to either adjacent or nonadjacent carbon atoms and situated in an eclipsed fashion. The boat form of cyclohexane has two forms of torsional strain. The first type is caused by the interaction of atoms or groups that are eclipsed on adjacent carbons. It occurs between the four lower hydrogen atoms on the four carbons at the bottom of the boat form of cyclohexane. The second type is caused by eclipsed atoms or groups on nonadjacent carbons. This occurs between the eclipsed hydrogen atoms of the two upper carbons of the boat form. These two types of torsional strain account for the higher energy states of the eclipsed cycloalkanes compared to the cycloalkanes with staggered arrangements. Because the chair form of cyclohexane does not have torsional strain, it is more stable and has a lower energy state than the boat form.
![]()