Covalent Bonding and Electronegativity
Covalent bonds form when atoms share electrons. This sharing allows each atom to achieve its octet of electrons and greater stability. Methane, CH 4, the simplest organic compound, contains covalent bonds. Carbon has four valence electrons, while hydrogen has one valence electron. By sharing these outer‐shell electrons, carbon and hydrogen complete their valence shells and become more stable. The duet of electrons on the hydrogen is isoelectronic with helium and forms a complete shell.
Polarity of bonds. In a pure covalent bond, the shared electrons are equally available to each of the atoms. This arrangement occurs only when two atoms of the same element bond with each other. Thus, the hydrogen molecule, H 2, contains a good example of a pure covalent bond. ![]()
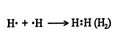
In most cases, the electrons in covalent bonds are not shared equally. Usually, one atom attracts the bonding electrons more strongly than does the other. This uneven attraction results in these electrons moving closer to the atom with the greater power of attraction. The resulting asymmetrical distribution of electrons makes one end of the molecule more electron rich, and it acquires a partial negative charge, while the less electron rich end acquires a partial positive charge. This difference in electron density causes the molecule to become polar, that is, to have a negative and a positive end.
The ability of an atom to attract electrons in a chemical bond is called the electronegativity of the atom. The electronegativity of an atom is related to its electron affinity and ionization energy. Electron affinity is the energy liberated by a gaseous atom when an electron is added to it. Ionization energy is the minimum amount of energy necessary to remove the most weakly bound electron from a gaseous atom.
Electronegativity level is normally measured on a scale that was created by Linus Pauling. On this scale, the more electronegative elements are the halogens, oxygen, nitrogen, and sulfur. Fluorine, a halogen, is the most electronegative with a value of 4.0, which is the highest value on the scale. The less electronegative elements are the alkali and alkaline earth metals. Of these, cesium and francium are the least electronegative at values of 0.7.
Elements with great differences in electronegativity tend to form ionic bonds. Atoms of elements with similar electronegativity tend to form covalent bonds. (Pure covalent bonds result when two atoms of the same electronegativity bond.) Intermediate differences in electronegativity between covalently bonded atoms lead to polarity in the bond. As a rule, an electronegativity difference of 2 or more on the Pauling scale between atoms leads to the formation of an ionic bond. A difference of less than 2 between atoms leads to covalent bond formation. The nearer the difference in electronegativity between atoms comes to zero, the purer the covalent bond becomes and the less polarity it has.
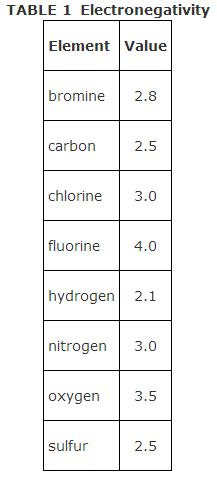
Carbon, with an electronegativity of 2.5, forms both low‐ and high‐polarity covalent bonds. The electronegativity values of elements commonly found in organic molecules are given in Table .
|
|
|
|
|
|
|
|