The concept of the atom was created by early Greek philosophers who believed that all matter was composed of indivisible particles. They called these particles atomos, meaning “uncuttable.” It wasn't until the early nineteenth century that John Dalton formulated a theory based on scientific investigation that characterized the nature of atoms. Further discoveries in the nineteenth and twentieth centuries led to the knowledge that atoms possess an internal structure of smaller subatomic particles.
Subatomic particles. The major subatomic particles were found to be protons, electrons, and neutrons. Protons are positively charged particles that have weight. Electrons are negatively charged particles of little weight, while neutrons are just slightly heavier than protons but have no charge. Investigations revealed that protons and neutrons are located in the central core, or nucleus, of the atom, while electrons exist outside of the nucleus in areas of high probability called orbits, or shells. Orbits are further divided into more precise regions of electron probability called orbitals, or subshells.
Niels Bohr proposed the concept of the solar‐system atom, in which the nucleus of the atom is like the sun and the electrons are like the planets, revolving in circular orbits. The farther an orbit is from the nucleus, the larger the orbit becomes and the more electrons it can hold.
Because all atoms are electrically neutral, the number of protons and electrons must be equal. Neutrons add weight but no charge to an atom, so additional neutrons do not change an element but merely convert it to one of its isotopic forms. The atomic number ( Z) of an atom is equal to the number of protons in the nucleus or the number of electrons in its orbits. The atomic mass ( A) is equal to the sum of the protons and neutrons in the atom. (A proton and neutron each have a mass of 1 atomic mass unit, while an electron has virtually no mass.)
Atoms are capable of both losing and gaining electrons to achieve a stable state. If an atom loses one or more electrons, it becomes a positively charged ion called a cation. If an atom gains one or more electrons, it becomes a negatively charged ion called an anion. The charge on an ion is equal to the number of electrons lost or gained.
Orbits and orbitals. Electrons fill orbits in an organized fashion based on energy factors. The order of electron fill‐in, called the aufbau buildup, is 1 s, 2 s, 2 p, 3 s, 3 p, 4 s, …, where the numerals represent the principal quantum number of the orbit, and the lowercase letters represent the orbitals within a given orbit. The numbering begins with 1 for the orbit closest to the nucleus of the atom. The lower the orbit number, the smaller the orbit size and fewer electrons the orbit can hold.
The first principal orbit is large enough to hold just two electrons in an s orbital. The second principal orbit is large enough to contain one s and three p orbitals, while the third principal orbit, which is larger still, contains an s orbital, three p orbitals, and five d orbitals. When electrons are added to equivalent orbitals, which are orbitals of the same principal level and type, one electron must occupy each equivalent orbital before any of these orbitals can contain two electrons. Thus carbon, Z = 6, has six electrons distributed in these orbitals: ![]()
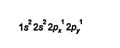
The orbitals can also be shown in the following fashion. In this diagram, the arrows represent electrons. Notice that single electrons are filling the 2 p orbitals one at a time and not pairing first in 2 p x . ![]()
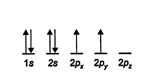
For two electrons to occupy the same orbital, they must have opposite spins, or paired spins, which generate orbital stability by creating opposite magnetic poles. Between equivalent orbitals, the spins of the electrons must be parallel, that is, spinning in the same direction, for the orbitals to be stable. Parallel spins create the same magnetic pole, causing repulsion between the orbitals. This repulsion gives the orbitals maximum separation and the greatest stability.
Orbitals within a given orbit have different shapes and sizes. The s orbitals are spherical, while the p orbitals are hourglass shaped. The s orbital is smaller than the p orbital.
|
|
|
|
|
|
|
|