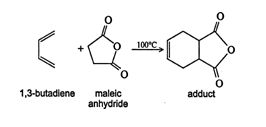
The Diels‐Alder reaction is a cycloaddition reaction between a conjugated diene and an alkene. This reaction produces a 1,4‐addition product. A typical example is the reaction of 1,3‐butadiene with maleic anhydride.
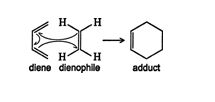
The Diels‐Alder reaction is favored by the presence of electron‐withdrawing groups on the diene and electron‐releasing groups on the dienophile, which is a group or bond that is attracted to a diene. The mechanism for the Diels‐Alder reaction shows that it does not run via a carbocation intermediate. Instead, this reaction proceeds by a pericyclic process, a mechanism of just one step, involving a cyclic redistribution of bonding electrons. ![]()
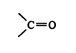
Simple alkenes and alkynes are not good dienophiles. A good dienophile generally has one or more electron‐withdrawing carbonyl groups
or cyano groups
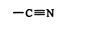
attached to it. Electron‐supplying groups
![]() on the diene make the electrons of the π system more available for reaction.
on the diene make the electrons of the π system more available for reaction.
Diels‐Alder stereochemistry. The Diels‐Alder reaction is very ste‐reospecific. The original stereochemistry of the diene and the dienophile are preserved during this syn‐addition reaction. An example of this stereospecificity is the reaction of 1,3‐butadiene with cis‐diethylmaleate.
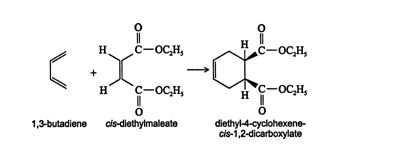
![]()
Because the reaction is basically a concerted cyclization, the diene must react in the cis conformation.
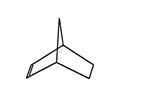
If the diene is a ring structure, the Diels‐Alder reaction produces a bicyclic ring system.
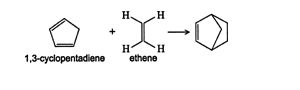
![]()
A bicyclic ring system has two carbon rings that share common sides. The previous diagram shows what appears to be a cyclohexene ring with a carbon bridge connecting the third and sixth carbons. In reality, this system is two five‐membered rings, a cyclopentene ring and a cyclopentane ring, which are sharing two sides (the “carbon bridge”). The structure of this molecule is