Cahn‐Ingold‐Prelog RS Notational System
Because enantiomers are different configurations of the same compound, a notational system had to be developed that would indicate the three‐dimensional arrangement of atoms at specific stereogenic centers. Such a system was devised by the chemists Cahn, Ingold, and Prelog. In this system, the substituents of a stereogenic center are ranked by atomic weight as dictated by a series of priority rules. A projection of the molecule is then viewed so that the group or atom of lowest priority is eclipsed by the stereogenic center. The ranking of the three remaining groups is then determined. If their rank from highest to lowest is in a clockwise direction, the configuration is R. On the other hand, if the rank declines in a counterclockwise direction, the configuration is S. The labels R and S come from the Latin words rectus, which means “right,” and sinister, meaning “left.” The right and left designations refer only to the order of atoms or groups about a stereogenic center. They do not refer to the direction in which plane‐polarized light is rotated by the molecule.
The direction of rotation of plane‐polarized light by a molecule is designated “d” or “+” for dextrorotatory compounds, which rotate plane‐polarized light to the right, and “l” or “+‐” for levorotatory compounds, which rotate plane‐polarized light to the left.
The following sequence rules summarize the Cahn‐Ingold‐Prelog notational system.
- Identify the four different atoms or groups attached to the stereogenic center.
- Rank the atoms or groups based on the priority rules (see the list following this one).
- Orient a projection of the molecule in space so that the group or atom of lowest rank is eclipsed by the stereogenic center.
- Determine the ranking of the remaining visible atoms or groups.
- If the ranking declines in a clockwise direction, the configuration is R; if the ranking declines in a counterclockwise direction, the configuration is S.
The priority rules rank atoms and groups based on atomic mass. The following list summarizes these rules.
- For the four atoms directly attached to the stereogenic center, the higher the atomic mass, the higher the rank.
- If two or more atoms directly attached to the stereogenic center have the same mass, work outward along the chains of the groups they are in, atom by atom, until a point of difference is reached. The rank is assigned at this point of difference, based on the difference in atomic mass.
- If a group contains multiple bonds, the doubly or triply bonded atoms are counted as two or three of those atoms, respectively. Thus the carbonyl group
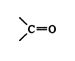
is considered to have two carbon‐oxygen bonds, one actual and one theoretical.
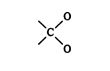
A cyano group

is considered to have three carbon‐nitrogen bonds, one actual and two theoretical. For comparison purposes, an actual bond ranks higher than a theoretical bond of the same type. For example, when ranking the cyano group against
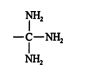
the displayed group takes priority, due to its three actual carbon‐nitrogen bonds. ![]()
The sequence rules can be illustrated by applying them to lactic acid.
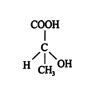
- The four atoms or groups around the stereogenic carbon are CH 3, H, COOH, and OH.
- The ranking based on atomic weights is oxygen > carbon > hydrogen. The ranking of the carbon‐containing groups is COOH > CH 3.
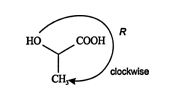
- The overall priority is thus OH > COOH > CH 3 > H. Viewing the molecule in such a way that the lowest ranked atom is eclipsed by the stereogenic center reveals that the remaining groups decline in rank in a clockwise direction. Thus, the stereogenic carbon has an R configuration.
Gaining the proper perspective for eclipsing the lowest ranked atom or group by the stereogenic carbon can be difficult. Try using an eclipsed downward sawhorse projection of the molecule. This projection allows rotation of the sawhorse axis so the atom or group of lowest rank can be placed at the bottom, thus allowing the point of view to be always from the top, or front, of the projection. Such a sawhorse projection must be drawn directly from a Fischer projection without any rotation. An eclipsed downward sawhorse projection of ( R)‐lactic acid can be drawn this way.
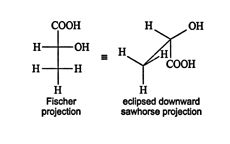
![]()
The projection can be abbreviated in this manner.
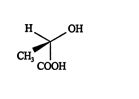
Rotation on the carbon‐carbon bond to put the hydrogen atom at the bottom of the projection looks like this.
![]()
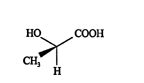
Viewing from the top eclipses the hydrogen atom.
![]()
The clockwise direction of the ranking is then easily seen.
RS notation can also be generated from Fischer projections; however, because Fischer projections are strictly two‐dimensional representations of three‐dimensional molecules, only certain manipulations are allowed. One such manipulation is the interchange of any two pairs of substituents. An interchange converts an enantiomer to its mirror image. Interchanging two pairs of substituents produces the original projection. Here is an example using a Fischer projection of ( R)‐lactic acid.
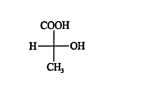
![]()
Verify its R or S configuration by using the following general steps (diagrams follow).
- Interchange the atom or group of lowest rank with the atom or group at the bottom of the Fischer projection.
- Interchange the atoms or groups on the remaining positions until rank order is established.
- Determine whether the ranking defines a clockwise or counterclockwise direction. If clockwise, the projection is an R configuration; if counterclockwise, it is an S configuration.
- The configuration of the original structure is determined by counting the number of interchanges made in step 2. If an odd number were made, the original configuration is opposite that of the projection. If an even number of interchanges were made, the original configuration and the projection are the same.
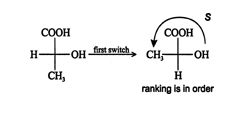
The ranking of this projection of ( R)‐lactic acid is S, and because it was arrived at after an odd number of switches (one), the original configuration is really R. The enantiomer of ( R)‐lactic acid is ( S)‐lactic acid, which has the configuration
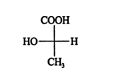
![]()
Try applying the four general steps to this configuration of lactic acid.
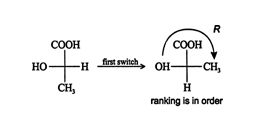
![]()
The final projection is R. However, this configuration was found after an odd number of exchanges (one); thus, the original configuration must be S.