Although many different types of nomenclature, or naming systems, were employed in the past, today only the International Union of Pure and Applied Chemistry (IUPAC) nomenclature is acceptable for all scientific publications. In this system, a series of rules has been created that is adaptable to all classes of organic compounds. For alkanes, the following rules apply.
1. Identify the longest continuous chain of carbon atoms. The parent name of the alkane is the IUPAC‐assigned name for the alkane of this number of carbon atoms (see Table ). Thus, if the longest chain of carbon atoms has six carbon atoms in it, the parent name for the compound is hexane.
2. Identify the substituent groups attached to the parent chain. A substituent group is any atom or group that has replaced a hydrogen atom on the parent chain.
3. Number the continuous chain in the direction that places the substituents on the lowest‐numbered carbon atoms.
4. Write the name of the compound. The parent name is the last part of the name. The name(s) of the substituent group(s) and the location number(s) precede the parent name. A hyphen separates the number associated with the substituent from its name. If two substituents are on the same carbon of the parent chain, the number of the carbon they are attached to is written before each substituent name. If the two substituents are identical, the numbers are both written before the substituent name, and the prefix “di” is added to the name. Substituent group names are placed in alphabetical order.
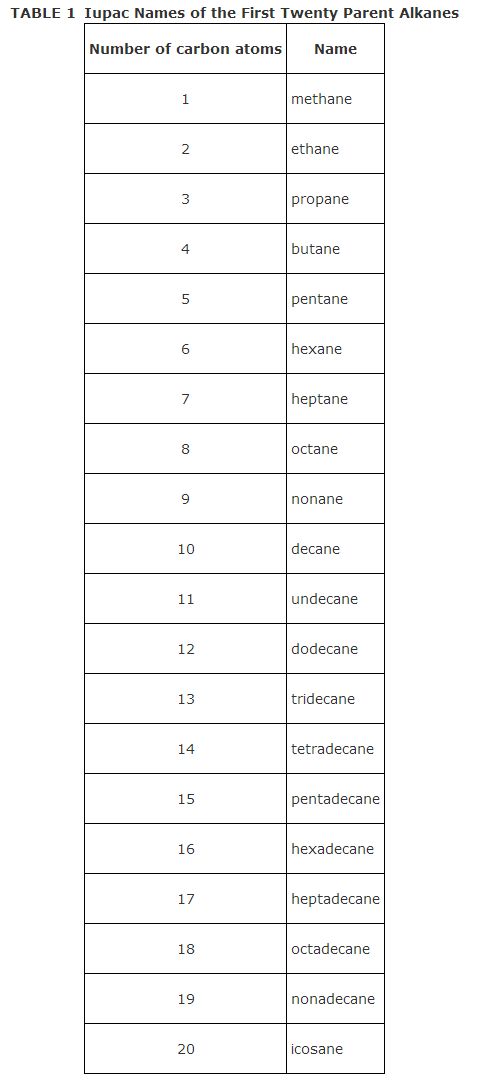
Applying the four nomenclature rules to the following compound
![]()
results in the name 2‐chloro‐3‐methylpentane. Notice that the parent name comes from the longest continuous carbon chain, which begins with the carbon of the CH 3 group at the bottom of the structural formula ( a) and goes to the carbon of the CH 3 group on the top right side of the formula ( b). This chain contains five carbon atoms, while the straight chain of carbons from left to right contains only four carbons. Starting the numbering from the top right carbon of the CH 3 group ( b) leads to 2,3 substitution, while numbering from the bottom right side CH 3 carbon ( a) leads to 3,4 substitution (which is incorrect). This alkane is referred to as a branched‐chain alkane because it contains an alkyl group off of the main chain.
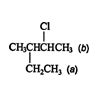
Applying the IUPAC nomenclature rules to a more complex alkane molecule
![]()
results in the name 5‐chloro‐2‐hydroxy‐4‐propylheptane. Notice that the names of the substituent groups are in alphabetical order.
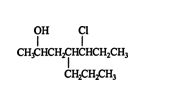
Finally, here is an example of a compound with a repeating substituent group. ![]()
This compound is called 2,4‐dichlorohexane.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|