Alkenes: Catalytic Addition of Hydrogen
Hydrogenation is the addition of hydrogen to an alkene. Although this reaction is exothermic, it is very slow. The addition of a metal catalyst, such as platinum, palladium, nickel, or rhodium, greatly increases the reaction rate. Although this reaction seems simple, it is a highly complex addition. The reaction takes place in four steps.
In the first step, a hydrogen molecule reacts with the metal catalyst. This reaction breaks the σ bond between the hydrogen atoms and creates weak hydrogen‐metal bonds. Next, the π bond of an alkene molecule contacts the metal catalyst. The π bond is destroyed and two weak carbon‐metal single bonds are created. Finally, the weakly bound hydrogen atoms transfer one at a time from the catalyst surface to the carbon atoms of the former alkene molecule, forming an alkane. Upon formation of the two new carbon‐hydrogen bonds, the alkane molecule can move away from the catalyst.
Because both of the added hydrogen atoms were bound to the surface of the catalyst, they normally approach the alkene molecule from the same side, or face. This approach of hydrogen atoms to the same face of an alkene molecule is called a syn addition. ![]()
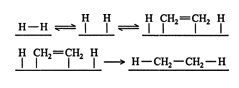
When hydrogen atoms approach alkene molecules from opposite sides, the reaction is called an anti addition. Anti addition most likely occurs when double‐bond isomerization occurs more rapidly than the catalytic addition of the second hydrogen in the hydrogenation.