An explanation of the various drawings, or projections, used to show the three‐dimensional structure of chemicals will help you understand the next section's discussion of enantiomers and diastereomers. The simplest drawing is called a Fischer projection. In this representation, the “backbone” atoms of a carbon chain are represented simply by a straight line, and the terminal carbons are written as groups. The atoms or groups bonded to the chain carbons are “attached” with perpendicular lines. Compare the structural formula and Fischer projection of 2‐bromo‐3‐chlorobutane.
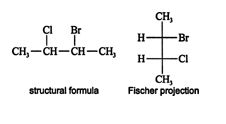
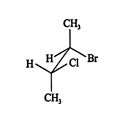
A second type of projection, a sawhorse projection, allows better visualization of the three‐dimensional geometry between adjacent carbon atoms. This projection is customarily used to show interactions between groups on adjacent carbon atoms in mechanisms. In a sawhorse projection, the backbone carbons are represented by a diagonal line, and the terminal carbons are shown in groups, just as in the Fischer projection. You can see in the next illustration that the top carbon group of the Fischer projection of 2‐bromo‐3‐chlorobutane has become the back carbon group of the sawhorse projection. ![]()
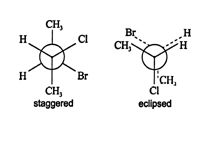
A sawhorse projection can reveal staggered and eclipsed conformations in molecules. The previous conformation of 2‐bromo‐3‐chlorobutane is referred to as a staggered conformer because the atoms and groups attached to each backbone carbon fit in the voids around the groups on the adjacent carbon. In an eclipsed conformer, the groups and atoms on adjacent carbons are in line with each other. ![]()
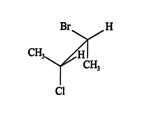
A third type of projection is called the Newman projection. This type of projection is used mainly to show interaction leading to stress between atoms or groups in three‐dimensional space due to steric crowding. In this representation, a molecule is viewed from one “end.” The “front” carbon of the backbone is represented by a dot, and the “back” carbon of the backbone is shown as a circle. Lines representing the bonds attaching the atoms and groups to the backbone carbons emanate from the dot and the circle at 120° angles. Compare the two conformations of 2‐bromo‐3‐chlorobutane in the Newman projection to the previous sawhorse and Fischer projections.
![]()