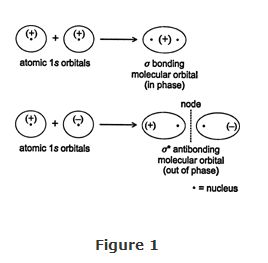
When two hydrogen atoms come together to form the hydrogen molecule, the atomic s orbitals of each atom are combined to form two molecular orbitals. One of these new orbitals is the result of the addition of the two atomic orbitals, while the other is created by a subtraction of these orbitals. In the addition, a reinforcement of the wave function occurs in the region between the two nuclei. Physically, this means the electron density increases in the area between the two nuclei. This increase in electron density causes a corresponding increase in the attraction of each positively charged nucleus for the negatively charged overlap region. It is this increased attraction that holds the hydrogen molecule together and creates the bonding molecular orbital. Because the bonding molecular orbital is generated from atomic s orbitals, it is called a σ (sigma) bonding molecular orbital.
The molecular orbital formed by the subtraction of the two wave functions has no electron density between the nuclei of the hydrogen atoms. This lack of electron density is caused by interference between the two out‐of‐phase wave functions. The lack of electron density between the nuclei results in the formation of a node. With no electron density between them, the hydrogen nuclei repel each other strongly, resulting in the formation of a high‐energy state called an antibonding orbital. Because this particular antibonding orbital is created from two atomic s orbitals, it is referred to as a σ * antibonding molecular orbital . The bonding and antibonding orbitals in the hydrogen molecule are illustrated in Figure . Note that the plus and minus symbols in the figure refer to wave phases and not electrical charge.
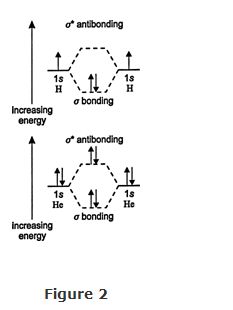
Like electrons in atomic orbitals, electrons in bonding orbitals must have paired spins; that is, the electrons must be spinning in opposite directions.
An energy diagram for the formation of the hydrogen molecule and the nonformation of a helium molecule are shown in Figure . The two electrons in a hydrogen molecule are paired in the lower‐energy σ bonding molecular orbital. No electrons (arrows, in the figure) occupy the σ * antibonding orbital. As long as a molecule has more electrons in the bonding orbital than in the antibonding orbital, it will be stable. In fact, in most stable molecules, the antibonding orbitals are vacant. Helium molecules do not exist because no driving force causes the helium atoms to bond. They have the same number of bonding and antibonding electrons, and thus achieve no greater stability as a molecule.
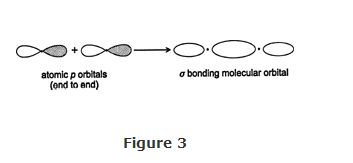
The hydrogen molecule illustrates that a σ bond must have a heavy electron density along an imaginary line between the two nuclei. Such a density can also exist in the end‐to‐end overlap of atomic p orbitals. As shown in Figure , the heaviest electron density lies along a line between the nuclei.
The overlap of atomic s orbitals with hybrid atomic orbitals and the overlap of two hybrid atomic orbitals can also result in σ bonds.
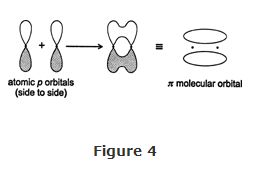
The side‐to‐side overlap of atomic p orbitals results in high electron density above and below an imaginary line between the nuclei. This density pattern, the π molecular orbital, leads to the formation of a π (pi) bond. This bond is much weaker than a α bond because the repulsion between the electronically unshielded nuclei leads to poor orbital overlap.