Enantiomers and Diastereomers
A Fischer projection is the most useful projection for discovering enantiomers. Compare the 2‐chlorobutane enantiomer structures in this diagram.
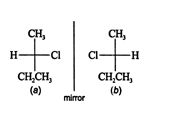
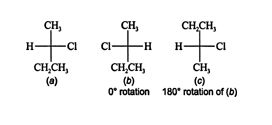
Rotating structure ( b) 180° in the plane of the paper, the only allowable rotation, does not lead to a form that is superimposable on structure ( a). Rotations of less than or more than 180° are not allowed because in a two‐dimensional projection, it is impossible to see the difference in the position of atoms that are located in front of or behind the plane.
![]()
Structures ( a) and ( b) are the only pair of enantiomers for 2‐chlorobutane.
The compound 2‐chloro‐3‐bromobutane has two stereogenic centers and a maximum of four enantiomers. Compare these two Fischer projections.
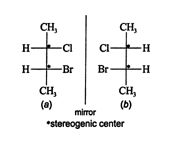
![]()
Structure ( b) cannot be superimposed on structure ( a) by rotating it in the plane of the page, so structures ( a) and ( b) are enantiomers. The additional two enantiomers are created by allowing rotation about one of the stereogenic centers while restricting rotation about the other. Structure ( c) is created by allowing rotation about the upper stereogenic center (carbon 2) of structure ( a).
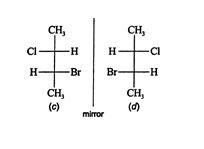
![]()
Notice that structure ( c) has a different configuration from structures ( a) and ( b). Structure ( d), the mirror image of ( c), cannot be superimposed on structure ( c) by rotating it in the plane of the page. Therefore, structures ( c) and ( d) are enantiomers. Any further rotation about the stereogenic centers creates a structure that is already drawn. For example, starting with structure ( a) and allowing rotation about the lower stereogenic center (carbon 3) generates structure ( d) again. This situation agrees with the maximum number of enantiomers predicted by the van't Hoff rule: 2 n = 2 2 = 4.
The relationship between the enantiomers of separate enantiomorphic pairs is called diastereoisomerism. For example, while structures ( a) and ( b), and ( c) and ( d), are enantiomers, the relationship of ( a) to ( c) is one of diastereoisomerism. They are not mirror images, so structure ( a) is a diastereomer of structures ( c) and ( d). Likewise, structure ( b) is a diastereomer of structures ( c) and ( d). In the same fashion, structures ( c) and ( d) are diastereomers of ( a) and ( b). Enantiomers have opposite configurations at all stereogenic centers, while diastereomers have the same configuration at one or more stereogenic centers but opposite configurations at others.
Optically inactive stereogenic centers ( meso forms). Some molecules are optically inactive even though they contain stereogenic centers. These compounds normally contain a plane of symmetry. The compound 2,3‐dichlorobutane should have four enantiomers because it has two stereogenic centers.
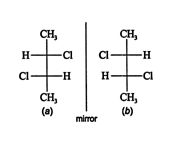
![]()
Structure ( b) cannot be superimposed on structure ( a) by rotating it in the plane of the page; thus, structures ( a) and ( b) are enantiomers. Rotation about the upper stereogenic center leads to structure ( c), which is a different configuration from ( a) and ( b).
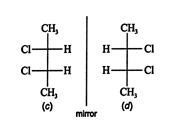
![]()
Structure ( d) is the mirror image of ( c). It can be superimposed on ( c) by rotating it 180°. Because these two structures are superimposable mirror images, they are not optically active, even though they contain two stereogenic centers. The reason for this lack of optical activity is the plane of symmetry through the center of the molecule.
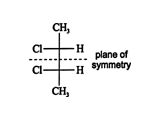
![]()
These types of molecules are called meso forms. In meso forms , the stereogenic centers are optically active, but due to the molecular symmetry, they rotate plane‐polarized light to the same degree but in opposite directions. This phenomenon results in an internal cancellation of optical activity.