Alkanes: Kinetics and Rate
Most reactions require the addition of energy. Energy is needed for molecules to pass over the energy barriers that separate them from becoming reaction products. These energy barriers are called the activation energy, or enthalpy of activation, of the reactions.
At room temperature, most molecules have insufficient kinetic energy to overcome the activation energy barrier so a reaction can occur. The average kinetic energy of molecules can be increased by increasing their temperature. The higher the temperature, the greater the fraction of reactant molecules that have sufficient energy to pass over the activation energy barrier. Thus, the rate of a reaction increases with increasing temperature.
The rate of a reaction also depends on the number of interactions between reactant molecules. Interactions increase in solutions of greater concentrations of reactants, so a reaction rate is directly proportional to the concentration of the reactants. The proportionality constant is called the rate constant for the reaction. Not every collision is effective in producing bond breakage and formation. For a collision to be effective, the molecules must have sufficient energy content as well as proper alignment. If all collisions were effective, every reaction would proceed with explosive force.
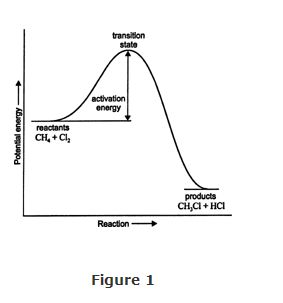
Activation energy. The change in structure of each of the reactants as a reaction proceeds is very important in organic chemistry. For example, in the reaction of methane and chlorine, the molecules of each substance must “collide” with sufficient energy, and the bonds within the molecules must be rearranged for chloromethane and hydrogen chloride to be produced. As reactant molecules approach each other, old bonds are cleaved, and new bonds are formed. The cleavage of bonds requires a lot of energy, so as the reaction occurs, the reactant molecules must remain in high‐energy states. When new bonds form, energy is released, and the resulting products possess less energy than the intermediates from which they were formed. When reactant molecules are at their maximum energy content (at the crest of the activation energy curve), they are said to be in a transition state. The energy necessary to drive the reactants to the transition state is the activation energy (Figure 1).
Many organic reactions involve more than one step. In such cases, the reactants may proceed through one or more intermediate stages (either stable or unstable arrangements), with corresponding transition states, before they finally form products (Figure 2).
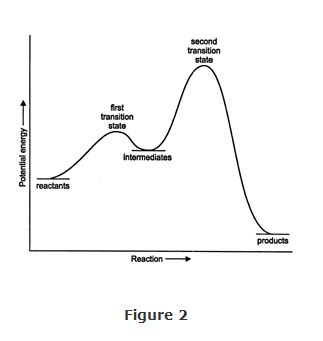
The overall rate of the reaction is determined, for the most part, by the transition state of highest energy in the pathway. This transition state, which is usually the slowest step, controls the rate of reaction and is thus called the rate‐determining step of the mechanism.
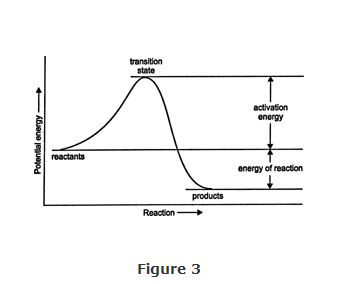
Energy of reaction. The energy of reaction is the difference between the total energy content of the reactants and the total energy content of the products (Figure 3). In ordinary organic reactions, the products contain less energy than the reactants, and the reactions are therefore exothermic. The energy of reaction has no effect on the rate of the reaction. The greater the energy of reaction, the more stable the products.
Effects of temperature on rate of reaction. The rates of organic reactions approximately double with each 10°C rise in temperature. A more quantitative relationship between reaction rate and temperature is given by the Arrhenius equation