Both isolated and conjugated dienes undergo electrophilic addition reactions. In the case of isolated dienes, the reaction proceeds in a manner identical to alkene electrophilic addition. The addition of hydrogen bromide to 1,4‐pentadiene leads to two products.
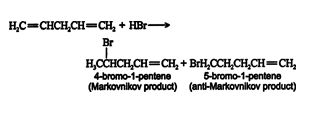
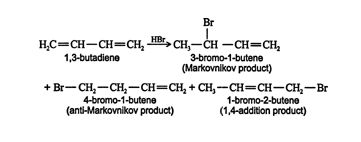
This reaction follows the standard carbocation mechanism for addition across a double bond. The addition of more hydrogen bromide results in addition across the second double bond in the molecule. In the case of conjugated dienes, a 1,4‐addition product forms in addition to the Markovnikov and anti‐Markovnikov products. Thus, in the addition of hydrogen bromide to 1,3‐butadiene, the following occurs. ![]()
The 1,4‐addition product is the result of the formation of a stable allylic carbocation. An allylic carbocation has the structure ![]()
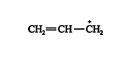
It is very stable because the charge on the primary carbon is delocalized along the carbon chain by movement of the π electrons in the π bond. This delocalization of charge by electron movement is called resonance, and the various intermediate structures are called resonance structures. However, according to resonance theory, none of the intermediate resonance structures are correct. The true structure is a hybrid of all the structures that can be drawn. The hybrid structure contains less energy and is thus more stable than any of the resonance structures. The more resonance structures that can be drawn for a given molecule, the more stable it is. The difference in energy between the calculated energy content of a resonance structure and the actual energy content of the hybrid structure is called the resonance energy, conjugation energy, or delocalization energy of the molecule. The allylic carbocation exists as a hybrid of two resonance structures. ![]()
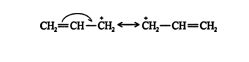
Because it is resonance stabilized, the allylic carbocation is much more stable than an ordinary primary carbocation. Resonance stability always leads to a more stable state than inductive stability. The hybrid structure for this ion is ![]()
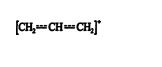
This structure shows the π‐electron movement throughout the conjugated system, with a resulting delocation of the positive charge through the system.
Understanding the allylic carbocation clarifies the mechanism for the addition to 1,3‐butadiene. ![]()
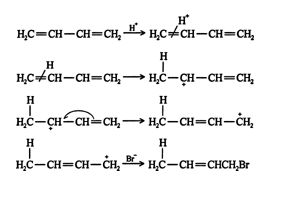
When other electrophiles are added to conjugated dienes, 1,4 addition also occurs. Many reactants, such as halogens, halogen acids, and water, can form 1,4‐addition products with conjugated dienes. Whether more 1,2 addition or 1,4 addition product is created depends largely on the temperature at which the reaction is run. For example, the addition of hydrogen bromide to 1,3‐butadiene at temperatures below zero leads mainly to the 1,2‐addition product, while addition reactions run at temperatures above 50°C with these chemicals produces mainly the 1,4‐addition product. If the reaction is initially run at 0°C and then warmed to 50°C or higher and held there for a period of time, the major product will be a 1,4 addition. These results indicate that the reaction proceeds along two distinct pathways. At high temperatures, the reaction is thermodynamically controlled, while at low temperatures, the reaction is kinetically controlled.
For the general reaction
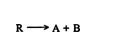
the high‐temperature, thermodynamically controlled reaction exists in an equilibrium state. ![]()
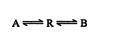
![]()
If B is more stable than A, B will be the major product formed. The rate of formation is immaterial because an increase in the forward reaction rate is mirrored by an increase in the reverse reaction rate. In reversible reactions, the product depends only on thermodynamic stability.
At low temperatures, the reaction is irreversible and no equilibrium is established because the products have insufficient energy to overcome the activation energy barrier, which separates them from the initial reactant. If A forms faster than B, it will be the major product. In irreversible reactions, the product depends only on the reaction rate and is therefore said to be kinetically controlled. Figure 1 is a reaction energy diagram that illustrates thermodynamically and kinetically controlled reactions.

Figure 1
The energy diagram of the reaction of 1,3‐butadiene with hydrogen bromide shows the pathways of the two products generated from the intermediate (Figure 2 ).

Figure 2