Alkenes: Hydration (Direct Addition of Water)
The addition of water to an alkene in the presence of a catalytic amount of strong acid leads to the formation of alcohols (hydroxy‐alkanes).
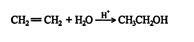
This reaction proceeds via a standard carbocation mechanism and follows the Markovnikov rule. The mechanism for the addition of water to ethene follows.
1. The hydrogen ion is attracted to the π bond, which breaks to form a σ bond with one of the double‐ bonded carbons. The second carbon of the original double‐bonded carbons becomes a carbocation. ![]()
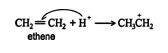
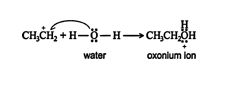
2.An acid‐base reaction occurs between the water molecule and the carbocation, forming an oxonium ion. ![]()
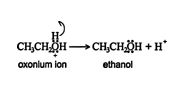
3. The oxonium ion stabilizes by losing a hydrogen ion, with the resulting formation of an alcohol.