Lewis Theory of Acids and Bases
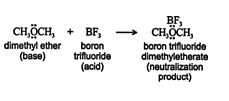
Both the Arrhenius and Brønsted‐Lowry theories of acids and bases define an acid as a hydrogen ion (proton) donor. In the Lewis theory, a base is any substance that can donate a pair of electrons to another compound. An acid then becomes any compound capable of accepting a pair of electrons from another substance. This theory greatly increases the number of chemicals considered to be acids and bases. For example, the reaction of boron trifluoride, BF 3, with dimethyl ether, CH 3OCH 3, is an acid‐base reaction. ![]()
|
|
|
|
|
|
|
|