The reaction of a halogen with an alkane in the presence of ultraviolet (UV) light or heat leads to the formation of a haloalkane (alkyl halide). An example is the chlorination of methane.
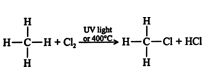
Experiments have shown that when the alkane and halogen reactants are not exposed to UV light or heat, the reaction does not occur. However, once a reaction is started, the light or heat source can be removed and the reaction will continue. The mechanism of the reaction explains this phenomenon.
Halogenation mechanism. In the methane molecule, the carbon‐hydrogen bonds are low‐polarity covalent bonds. The halogen molecule has a nonpolar covalent bond. UV light contains sufficient energy to break the weaker nonpolar chlorine‐chlorine bond (∼58 kcal/mole), but it has insufficient energy to break the stronger carbon‐hydrogen bond (104 kcal/mole). The fracture of the chlorine molecule leads to the formation of two highly reactive chlorine free radicals (chlorine atoms). A free radical is an atom or group that has a single unshared electron. ![]()
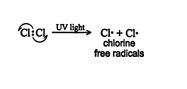
The bond that is ruptured is said to have broken in a homolytic fashion; that is, each of the originally bonded atoms receives one electron. This initial reaction is called the initiation step of the mechanism. The chlorine free radicals that form are in a high‐energy state and react quickly to complete their octets and liberate energy. Once the high‐energy chlorine free radicals are formed, the energy source (UV light or heat) can be removed. The energy liberated in the reaction of the free radicals with other atoms is sufficient to keep the reaction running.
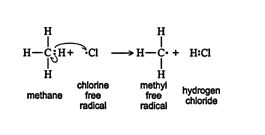
When a chlorine free radical approaches a methane molecule, a homolytic fission of a carbon‐hydrogen bond occurs. The chlorine free radical combines with the liberated hydrogen free radical to form hydrogen chloride and a methyl free radical. This is called a propagation step, a step in which both a product and a reactive species, which keeps the reaction going, are formed. ![]()
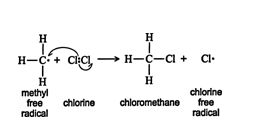
A second propagation step is possible. In this step, a methyl free radical reacts with a chlorine molecule to form chloromethane and a chlorine free radical. ![]()
When a reaction occurs between free radicals, a product forms, but no new free radicals are formed. This type of reaction is called a termination step because it tends to end the reaction. There are several termination steps in the chlorination of methane.
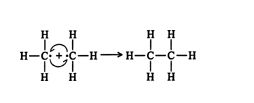
1. A methyl free radical reacts with a chlorine free radical to form chloromethane. ![]()
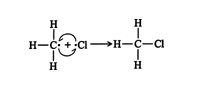
2. Two methyl free radicals react to form ethane. ![]()
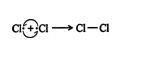
3. Two chlorine free radicals react to form a chlorine molecule. ![]()
To summarize, this free‐radical chain reaction initially contains few free radicals and many molecules of reactants. As the reaction proceeds, the number of free radicals increases, while the number of reactant molecules decreases. Near the end of the reaction, many more free radicals exist than reactant molecules. At this stage of the overall reaction, termination steps become the predominant reactions. All of the halogenation mechanism reactions occur very rapidly, and the formation of the products takes only microseconds.