Cyclohydrocarbons: Reactions
Due to angle strain, the bonds in three‐ and four‐membered carbon rings are weak. Because of these weak bonds, cyclopropane and cyclobutane undergo reactions that are atypical of alkanes. For example, cyclopropane reacts with halogens dissolved in carbon tetra‐chloride to form dihaloalkanes.
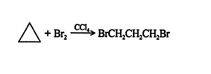
Under similar conditions, straight‐chain propane does not react.
In general, cycloalkanes undergo the normal reactions of the aliphatic alkanes (the straight‐chain and branched‐chain alkanes). Thus, cyclopentane will react with halogens in ultraviolet light to form halosubstituted cycloalkanes.
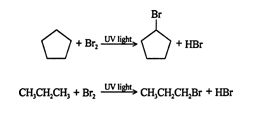
![]()
Cycloalkenes and cycloalkynes undergo the ordinary addition reactions of alkenes and alkynes. Cyclopropene, cyclopropyne, cyclobutene, and cyclobutyne also undergo ring‐opening reactions.