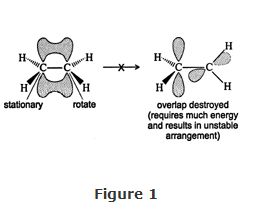
Nonrotation Around Multiple Bonds
Double and triple bonds are referred to as multiple bonds. These bonds are composed of a σ bond and either one or two π bonds. Because the π bonds are created by the side‐to‐side overlap of atomic p orbitals, any rotation around the σ bond results in the destruction of all of the π bonds. The effect on the double bond of ethene if rotation is attempted around the σ bond is shown in Figure .