Alkenes are normally named using the IUPAC system. The rules for alkenes are similar to those used for alkanes. The following rules summarize alkene nomenclature.
1. Identify the longest continuous chain of carbon atoms that contains the carbon‐carbon double bond. The parent name of the alkene comes from the IUPAC name for the alkane with the same number of carbon atoms, except the ‐ane ending is changed to ‐ene to signify the presence of a double bond. For example, if the longest continuous chain of carbon atoms containing a double bond has five carbon atoms, the compound is a pentene.
2. Number the carbon atoms of the longest continuous chain, starting at the end closest to the double bond. Thus, is numbered from right to left, placing the double bond between the second and third carbon atoms of the chain. (Numbering the chain from left to right incorrectly places the double bond between the third and fourth carbons of the chain.)
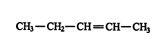
![]()
3. The position of the double bond is indicated by placing the lower of the pair of numbers assigned to the double‐bonded carbon atoms in front of the name of the alkene. Thus, the compound shown in rule 2 is 2‐pentene.
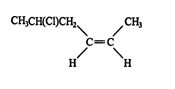
4. The location and name of any substituent molecule or group is indicated. For example, is 5‐chloro‐2‐` hexene.
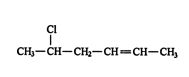
![]()
5. Finally, if the correct three‐dimensional relationship is known about the groups attached to the double‐ bonded carbons, the cis or trans conformation label may be assigned. Thus, the complete name of the compound in rule 4 (shown differently here) is cis‐5‐chloro‐2‐hexene.