Halogenation is the addition of halogen atoms to a π‐bond system. For example, the addition of bromine to ethene produces the substituted alkane 1,2‐dibromoethane.
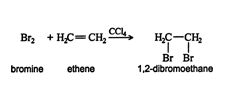
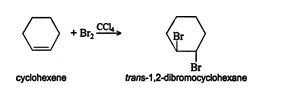
The reaction proceeds via a trans addition, but because of the free rotation possible around the single bond of the resulting alkane, a trans product cannot be isolated. If, however, the original alkene structure possesses restricted rotation due to a factor other than a double bond, a trans‐addition product can be isolated. For instance, ring structures possess restricted rotation. In a ring structure, the carbon backbone is arranged so there is no beginning or ending carbon atom. If cyclohexene, a six‐carbon ring that has one double bond, is halogenated, the resulting cycloalkane is trans substituted.
![]()
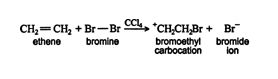
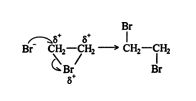
Mechanism and stereochemistry of halogenation. Alkenes and halogens are nonpolar molecules. However, both types of molecules, under proper conditions, can undergo induced‐dipole formation, which leads to the generation of forces of attraction between the molecules.
![]()
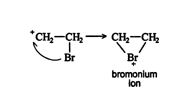
The bromoethyl carbocation that forms mid reaction in this example is often internally stabilized by cyclization into a three‐membered ring containing a positively charged bromine atom (bromonium ion).
![]()
This intermediate is more stable than the corresponding linear carbocation because all the atoms have a complete octet of electrons.
The bromonium ion shares the electrons in the carbon‐bromine covalent bond unevenly, with the overlap region being closer to the more electronegative bromine. This generates a partial positive charge (δ +) on the carbon atoms of the ring. The charge delocalization stabilizes the ring structure, and the resulting partial positive charges on the carbon atoms attract the nucleophilic bromide ion.
![]()
The second bromide ion must approach a partially positive carbon atom from the side of the carbocation opposite where the bromonium ion attached. The reason for this is that the bromonium ion blocks access to the carbon atoms along an entire side, due to bond formation with the two carbon atoms. Such blocking is referred to as steric hindrance. Because of steric hindrance, only a trans addition is possible.