First Phase of the TCA Cycle
Entry of 2‐carbon units is carried out by pyruvate dehydrogenase and citrate synthase in the first phase of the TCA cycle. Pyruvate from glycolysis or other pathways enters the TCA cycle through the action of the pyruvate dehydrogenase complex, or PDC. PDC is a multienzyme complex that carries out three reactions:
- Removal of CO 2 from pyruvate . This reaction is carried out by the pyruvate decarboxylase (E1) component of the complex. Like yeast pyruvate decarboxylase, responsible for the production of acetaldehyde, the enzyme uses a thiamine pyrophosphate cofactor and oxidizes the carboxy group of pyruvate to CO 2. Unlike the glycolytic enzyme, acetaldehyde is not released from the enzyme along with CO 2. Instead, the acetaldehyde is kept in the enzyme active site, where it is transferred to Coenzyme A.
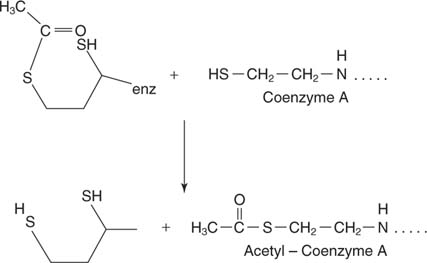
- Transfer of the 2‐carbon unit to Coenzyme A. This reaction is carried out by the dihydrolipamide transacetylase (E2) component of the complex. Lipoic acid is an 8‐carbon carboxylic acid with a disulfide bond linking the 6 and 8 carbons:

Lipoic acid is bound in an amide linkage with the terminal amino group of a lysine side chain. This long side chain means that the disulfide group of lipoic acid is capable of reaching several parts of the large complex. The disulfide reaches into the adjacent E 2 portion of the complex and accepts the 2‐carbon unit on one sulfur and a hydrogen atom on the other. Therefore, the oxidized disulfide is reduced, with each sulfur accepting the equivalent of one electron from the pyruvate carboxylase subunit.
The lipoic‐acid‐bound acetyl group is transferred to another thiol, the end of Coenzyme A, a cofactor composed of an ADP nucleotide bound through its phosphates to pantothenic acid, a vitamin, and finally, an amide with mercaptoethylamine. The acetyl group on lipoic acid is transferred to the free thiol (‐SH) group of Coenzyme A, leaving the lipoic acid with two thiols:
Acetyl‐CoA is the substrate for formation of citrate to initiate the TCA cycle.
- Regeneration of the disulfide form of lipoic acid and release of electrons from the
complex. This reaction is carried out by the third component of the pyruvate dehydrogenase complex—dihydrolipoamide dehydrogenase (E 3). This component contains a tightly bound cofactor—flavin adenine nucleotide, or FAD. FAD can function as a one‐ or two‐ electron acceptor. In the reaction catalyzed by E 3, FAD accepts two electrons from the reduced lipoic acid, leaving the side chain in a disulfide form. The reduced FADH 2 is regenerated by transferring two electrons from FADH 2 to NAD (see Figure 1 ).

Figure 1
In summary, the reactions of the complex are:
- E 1: pyruvate + TPP → CO 2 + hydroxyethyl‐TPP
- E 1: TPP + pyruvate
 CO 2 + E1: H
CO 2 + E1: H  TPP
TPP
- E 1 + E 2: hydroxyethyl‐TPP + lipoic acid → acetyl‐lipoic acid + TPP
- E 2: acetyl‐lipoic acid + Coenzyme A → acetyl‐CoA + E 2: lipoic acid reduced
- E 2: lipoic acid reduced + E 3 FAD → E 2 <: lipoic acid + E 3: FADH 2
- E 3: FADH 2 + NAD → E 3: FAD + NADH + H +
Summing up the equations and canceling out the intermediates that appear on both sides of the summed equation yields the overall reaction:
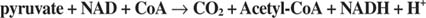
Acetyl‐CoA reacts with a 4‐carbon dicarboxylic acid—oxaloa‐ cetate—in the second entry reaction of the TCA cycle, which is catalyzed by citrate synthase. In organic chemistry terms, the reaction is an aldol condensation. The methyl group of acetyl‐CoA donates a proton to a base in the active site of the enzyme, leaving it with a negative charge. The carbonyl carbon of oxaloacetate is electron‐poor and is thereby available for conjugation with the acetyl group, making citroyl‐CoA. Hydrolysis of this intermediate releases free Co‐A and citrate (see Figure 2).

Figure 2
Citrate is not a good substrate for decarboxylation. Decarboxylation is usually carried out on alpha‐keto acids (like pyruvate, above) or alpha‐hydroxy acids. Conversion of citrate into an alpha‐hydroxy acid involves a two‐step process of water removal (dehydration), making a double bond, and readdition (hydration) of the intermediate—aconitate as Figure 3 shows. The enzyme responsible for this isomerization is aconitase.

Figure 3
Oxidative decarboxylation
Oxidative decarboxylation of isocitrate and alpha‐ketoglutarate releases CO 2 and reducing equivalents as NADH. The first decarboxylation is a consequence of the oxidation of isocitrate by transfer of two electrons to NAD, catalyzed by isocitrate dehydrogenase. Removal of the pair of electrons from the hydroxyl group results in an alpha‐keto form of isocitrate, which spontaneously loses CO 2 to make alpha‐ketoglutarate (see Figure 4). This 5‐carbon dicarboxylic acid is a participant in numerous metabolic pathways, as it can be easily converted into glutamate, which plays a key role in nitrogen metabolism.

Figure 4
Decarboxylation and oxidation of alpha‐ketoglutarate is carried out by a large multienzyme complex. Both in the overall reaction it catalyzes and in the cofactors used to carry them out—the alpha‐ketoglutarate/dehydrogenase complex (alpha‐KGDC)—it is similar to the reaction scheme of the pyruvate dehydrogenase (PDC) complex (see Figure 5 ).



Figure 5
Like the pyruvate dehydrogenase complex, the alpha‐ketoglutarate dehydrogenase complex has three enzymatic activities, and the same cofactors. As might be expected, the primary sequences of the proteins are highly similar, indicating that they diverged from a common set of ancestral proteins.
The result of this second phase of the TCA cycle is the release of two carbons from citrate. Thus, the equivalent of one mole of pyruvate has been converted to CO 2 by this point in the cycle, although the two carbons of acetyl‐CoA are still found in succinyl‐CoA. The two carbons released as CO 2 are derived from the original oxaloacetate involved in the citrate synthase reaction.
The third phase of the TCA cycle
Succinyl‐CoA is hydrolyzed and the 4‐carbon dicarboxylic acid is converted back to oxaloacetate in the third phase of the TCA cycle. Succinyl‐CoA is a high‐energy compound, and its reaction with GDP (in animals) or ADP (in plants and bacteria) and inorganic phosphate leads to the synthesis of the corresponding triphosphate and succinate—a 4‐carbon dicarboxylic acid. The substrate‐level phosphorylation is catalyzed by succinyl‐CoA synthetase:

(Figure 6
shows the reaction catalyzed by this enzyme.)

Figure 6
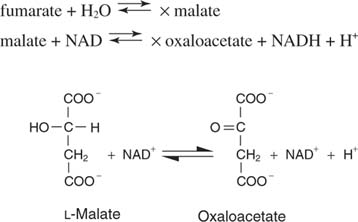
Succinate, the 4‐carbon saturated precursor to oxaloacetate, then undergoes three successive reactions to regenerate oxaloacetate. The first step is carried out by succinate dehydrogenase, which uses FAD as an electron acceptor, as Figure shows.

Fumarate is the trans isomer of the dicarboxylic acid.
Water is added across the double bond in the next step, catalyzed by fumarase, to give malic acid, or malate. Finally, malate dehydrogenase removes the two hydrogens from the hydroxyl carbon to regenerate the alpha‐keto acid, oxaloacetate: