The energy‐yielding steps of glycolysis involve reactions of 3‐carbon compounds to yield ATP and reducing equivalents as NADH. The first substrate for energy production is glyceraldehyde‐3‐phosphate, which reacts with ADP, inorganic phosphate, and NAD in a reaction catalyzed by the enzyme glyceraldehyde‐3‐phosphate dehydrogenase:
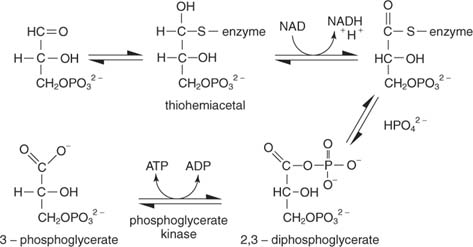
The reaction has several steps. In the first, a thiol carbon of the enzyme attacks the aldehyde carbon of glyceraldehyde‐3‐phosphate to make a thiohemiacetal intermediate. (Recall from organic chemistry that carbonyl carbons are electron‐poor and therefore can bond with nucleophiles, including thiols from which the proton is removed.) Next, NAD accepts two electrons from the enzyme‐bound glyceraldehyde‐3‐phosphate. The aldehyde of the substrate is oxidized to the level of a carboxylic acid in this step. Inorganic phosphate then displaces the thiol group at the oxidized carbon (carbon 1 of glyceraldehyde‐3‐phosphate) to form 1,3‐bisphosphoglycerate:
The next step is the transfer of phosphate from 1,3‐bisphosphoglycerate to ADP, making ATP, catalyzed by phosphoglycerate kinase.
This phase of glycolysis brings the energy balance from glucose back to zero. Two ATP phosphates were invested in making fructose‐1,6‐bisphosphate and two are now returned, one from each of the 3‐carbon units resulting from the aldolase reaction.
The next reaction is the isomerization of 3‐phosphoglycerate to 2‐phosphoglycerate, catalyzed by phosphoglycerate mutase:
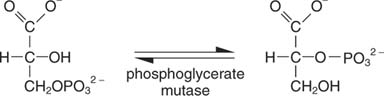
The reaction is pulled to the right by further metabolism of 2‐phosphoglycerate. First, the compound is dehydrated by the removal of the hydroxyl group on carbon 3 and a proton from carbon 2, leaving a double bond between carbons 2 and 3. The enzyme responsible for this step is a lyase, enolase:
 \
\

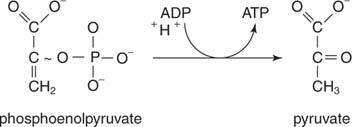
Enols are usually not as stable as keto compounds. Phosphoenol pyruvate, the product of enolase, is unable to tautomerize to the keto form because of the phosphate group. (Recall from organic chemistry that tautomers are compounds that react as if they were made up of two components, differing only in the placement of a substituent, like a hydrogen atom.) Therefore, there is a large negative free energy change associated with release of the phosphate; phosphate release allows the formation of the keto tautomer—that, is, of pyruvate. This free energy change is more than enough to phosphorylate ADP to make ATP in the reaction catayzed by pyruvate kinase
: 
This reaction, which is highly favored thermodynamically, brings glycolysis into positive energy balance because two ATP bonds are made—one from each of the 3‐carbon units from glucose.
The overall reaction of glycolysis is therefore:
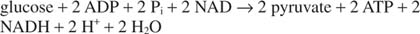
This still leaves one bit of unfinished business. The NAD converted to NADH in the glyceraldehyde‐3‐phosphate dehydrogenase reaction must be regenerated; otherwise glycolysis could not continue for very many cycles. This regeneration can be done anaerobically, with the extra electrons transferred to pyruvate or another organic compound, or aerobically, with the extra electrons transferred to molecular oxygen, with the generation of more ATP molecules.
The simplest way of regenerating NAD is simply to transfer the electrons to the keto group of pyruvate, yielding lactate, in the reaction catalyzed by lactate dehydrogenase. This reaction takes place in animal cells, especially muscle cells, and is carried out by lactic acid bacteria in the fermentation of milk to yogurt.
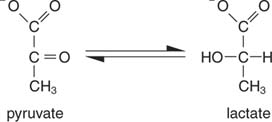
The formation of lactate oxidizes the two NADH molecules into NAD; therefore, the glycolytic breakdown of one molecule of glucose becomes: 
Ethanol
Ethanol results from the decarboxylation of pyruvate and the reduction of acetaldehyde. Yeasts and other organisms that produce ethanol use a two‐step reaction sequence. First, pyruvate decarboxylase releases CO 2 to make acetaldehyde. Then alcohol dehydrogenase transfers a pair of electrons from NADH to the acetaldehyde, resulting in ethanol
. 
When ethanol is produced, the reaction of glycolysis becomes:
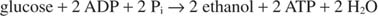
The preceding equation explains some traditional winemaking practices. Grapes with the highest sugar content generally make the best wine. On the other hand, unfortified wines have a maximum alcohol content of about 14%, because ethanol inhibits growth and fermentation at that concentration.
The alcohol dehydrogenase reaction occurs in the opposite direction when ethanol is consumed. Alcohol dehydrogenase is found in liver and intestinal tissue. The acetaldehyde produced by liver alcohol dehydrogenase may contribute to short ‐ and long‐term alcohol toxicity. Conversely, different levels of intestinal alcohol dehydrogenase may help explain why some individuals show more profound effects after only one or two drinks than others. Apparently, some of the ethanol consumed is metabolized by intestinal alcohol dehydrogenase before it reaches the nervous system.
Pyruvate to acetyl-Coenzyme A: The entry point into the TCA cycle
Pyruvate can be oxidatively decarboxylated to form acetyl‐Coenzyme A, which is the entry point into the TCA cycle. Louis Pasteur noted in the 1860s that the consumption of glucose by yeast is inhibited by oxygen. This is a regulatory phenomenon, whereby high levels of ATP formed by oxidative metabolism lead to the allosteric inhibition of crucial enzymes in the glycolytic pathway. How does oxidative metabolism form more ATP than fermentation does? Because the carbons from glycolysis are fully oxidized to CO 2 through the TCA cycle. The reducing equivalents produced by these oxidations are transferred to molecular oxygen, forming H 2O. More free energy is available from the complete oxidation of carbons to CO 2 than from the partial oxidations and reductions resulting from anaerobic glycolysis.