ATP synthesis involves the transfer of electrons from the intermembrane space, through the inner membrane, back to the matrix. The transfer of electrons from the matrix to the intermembrane space leads to a substantial pH difference between the two sides of the membrane (about 1.4 pH units). Mitchell recognized that this represents a large energy difference, because the chemiosmotic potential is actually composed of two components. One component is the difference in hydrogen ion concentration (pH out ‐ pH in), symbolized by the term ΔpH. The other component follows from the fact that protons are positively charged, so there is a difference in electrical potential symbolized by the term ΔΨ. The proton gradient results in a state where the intermembrane space is positive and acidic relative to the matrix. The shorthand for this situation is: positive out, negative in; acidic out, basic in. Quantitatively, the energy gradient across the membrane is the sum of the energies due to these two components of the gradient:
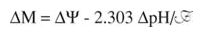
The combination of the two components provides sufficient energy for ATP to be made by the multienzyme Complex V of the mitochondrion, more generally known as ATP synthase. (See Figure 1.)
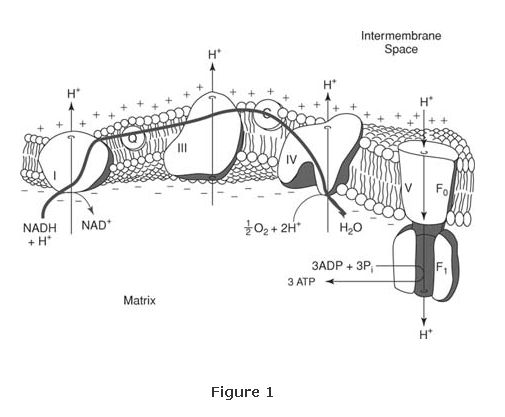
ATP synthase contains a membrane‐spanning domain, sometimes known as the F 0 subunit, and a knobby protrusion that extends into the matrix, the F 1 subunit. The mechanism of ATP synthase is not what one would naively predict. The F 1 ATP synthase subunit can perform its ligase function (making ATP from ADP and phosphate) without proton flow into the matrix; however, release of the ATP requires flow of protons through the membrane.
The existence of ATP synthase implies that electron transport and ATP synthesis are not directly linked. This is borne out by two experimental observations: An artificial proton gradient can lead to ATP synthesis without electron transport, and molecules termed uncouplers can carry protons through the membrane, bypassing ATP synthase. In this case, the energy of metabolism is released as heat. One such uncoupler is the compound dinitrophenol, shown in Figure . Dinitrophenol is a weak acid that is hydrophobic enough to be soluble in the inner membrane. It is protonated in the intermembrane space and deprotonated on the matrix side of the membrane. Because no ATP is made, energy from food is not available for fat synthesis. Indeed, dinitrophenol was used as a diet drug until side effects, including liver toxicity, led to its being withdrawn from the market.
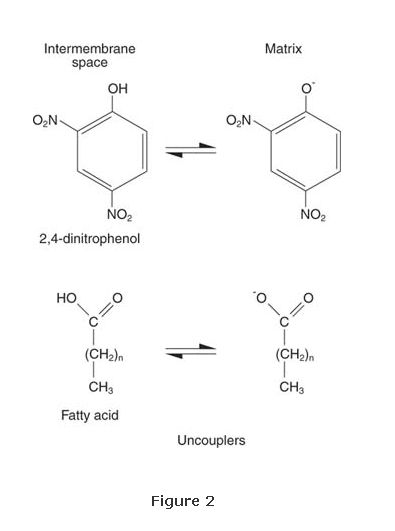
Fatty acids are also uncouplers—weak acids that can cross the inner membrane. In human infants, body heat can be generated by so‐called brown fat tissues at the base of the neck. The fat appears brown because it contains a high number of mitochondria, and the cytochromes give it a brownish‐red appearance. These mitochondria are in a naturally uncoupled state. Fat is oxidized but very little ATP is made; instead, the metabolic energy is converted to heat so that the brain can be kept warm and functioning. Regrettably, the brown fat tissue is lost with age, so adult humans can't burn off their excess calories so easily and naturally.