Oxidative Phosphorylation
The reducing equivalents from glycolysis, the Krebs cycle, or other catabolic pathways are carried by coenzymes, particularly NAD, and to some extent FAD. The coenzymes then need to be reoxidized so that the coenzymes can be used again. In anaerobic metabolism, the terminal electron acceptor is a carbon‐containing compound, such as pyruvate or acetaldehyde. The Krebs cycle releases carbon as CO 2, which can be reduced, but only by a reductant stronger than NADH. In aerobic metabolism, the terminal electron acceptor is oxygen, O 2, which is reduced to water:
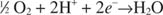
This reaction is highly exergonic. The energy of oxidation is the same, whether the reaction occurs in a fire or in a cell. The difference is that the reaction in a cell occurs in a controlled fashion, in small steps. The purpose of the reactions in the respiratory chain leading from NADH to oxygen is to conserve the energy of oxidation and convert it to ATP. The reducing equivalents from NADH are transferred through a series of membrane‐bound proteins, the cytochromes. As the electrons pass through the cytochromes, hydrogen ions, or protons, are released on one side of the membrane, leading to an electrical potential across the membrane. The protons flow across the membrane, and the energy associated with this electrical flow is converted to ATP. This overall process by which reducing equivalents are used to make ATP is known as oxidative phosphorylation. The process of proton flow leading to ATP synthesis is known as the chemiosmotic mechanism.
Oxidative phosphorylation occurs on membranes. In bacteria, chemiosmotic ATP synthesis occurs at the cytoplasmic membrane. In plant and animal cells, these reactions occur in the mitochondrion, a double‐membraned organelle (Figure 1 ). The ancestor of mitochondria was a bacterial cell incorporated into a nucleated cell, which subsequently lost much (although not all) of its DNA. Most mitochondrial proteins are encoded by nuclear DNA. Some respiratory proteins, along with mitochondrial ribosomal RNA and transfer RNAs, are encoded by mitochondrial DNA.

Figure 1
The outer membrane of the mitochondrion contains a large number of pores, so that molecules with molecular weights less than 1,000 can pass from the cytoplasm into the intermembrane space without any specialized transport mechanisms. This means, for example, that NADH, ADP, and inorganic phosphate can reach the intermembrane space from the cytoplasm while NAD and ATP can reach the cytoplasm. The inner membrane is much less permeable, in part due to the presence of a specialized membrane lipid known as cardiolipin (meaning heart lipid—cardiac cells have a large number of mitochondria). The inner membrane of the mitochondrion is highly folded into cristae, so that it has an interleaved appearance in the electron microscope.
Inside the inner mitochondrial membrane is the space called the matrix. The matrix contains DNA, the apparatus for mitochondrial protein synthesis, and the enzyme systems for the TCA cycle and fatty acid oxidation. (Note that the TCA cycle enzymes are also located in the cytoplasm, because they are involved in many other metabolic transactions in the cell.) Because generation of NADH for oxidative phosphorylation occurs in the matrix, and because the inner membrane is so impermeable, there must be a large number of specific transport systems to allow small molecules to reach the site where they are catabolized. Finally, many mitochondrial proteins are made by cytoplasmic ribosomes, encoded by nuclear DNA. This means that there must be protein transporting systems to bring these macromolecules into the matrix.
The energy of oxidation
The energy of oxidation is given by the redox potential of the reaction. If a piece of copper wire is placed in a solution of zinc sulfate, nothing happens, but if zinc metal is placed in a solution of copper sulfate, the zinc metal is corroded. Simultaneously, the blue color of the copper sulfate solution disappears, and metallic copper is deposited on the surface of the remaining zinc metal:
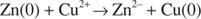
The reaction is the transfer of electrons from zinc to copper ions:
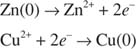
The overall reaction is the sum of these two half‐reactions. The reaction can occur even if the half reactions (that is, the zinc metal and copper ions) are in different containers, as long as the two half reactions are connected electrically, as in Figure 2.

Figure 2
The force associated with electron flow is the voltage. In Figure , the voltage can be measured, either by a voltmeter (similar to a battery tester), or by applying an opposing voltage to the wire connecting the zinc and copper electrodes. The amount of voltage required to cancel out the flow of electrons between the electrodes is called the potential of the Zn/Cu cell, designated E. The voltage of a cell depends on the concentrations (more accurately, the activities) of the ions. Chemists refer reaction energies to a standard state. In this case, the standard state is the one where the ions are present at 1 Molar concentration. The solid metals are always given the activity of 1. In the standard state, the potential of the Zn/Cu cell shown in Fig 11‐2 is about 1.1 volts; this means that it can be stopped by application of a direct current from a battery of 1.1 volts opposing the direction of spontaneous electron flow.
The reduction potential is related to the standard free energy change associated with reduction by the equation:

where n is the number of electrons involved in the reaction,  is the Faraday of a constant having the value 23.06 kcal Volts −1 mol −1 (96.5 kJ mol −1 V −1), and E o′ is the standard reduction potential for the two half‐reactions. Like other biochemical free‐energy changes, the standard reduction potential is determined at pH = 7.0.
is the Faraday of a constant having the value 23.06 kcal Volts −1 mol −1 (96.5 kJ mol −1 V −1), and E o′ is the standard reduction potential for the two half‐reactions. Like other biochemical free‐energy changes, the standard reduction potential is determined at pH = 7.0.
The standard reduction potential of a reaction is the sum of the two half‐reactions. For example, the standard free energy change associated with the reduction of pyruvate to lactate:

is the sum of the two half‐reactions:

NADH is oxidized to NAD, so the second half‐reaction must be reversed:

The standard reduction potential of the reaction is therefore the sum of the two half‐reactions:

This corresponds to the free energy change of:

Biochemical reduction and concentration-dependency
This dependence is given by the Nernst equation:
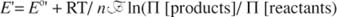
In the preceding reaction:
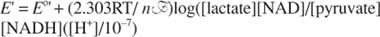
This is the same form as the dependence of free energy on the concentration of the reductants and products. The term ([H +]/10 –7) accounts for the fact that the standard state for biochemical reactions is at pH equal to 7.0.
The oxidation of NADH
The oxidation of NADH by molecular oxygen provides a large amount of free energy for ATP synthesis. The reduction potential of oxygen is +0.82 V; as can be seen in the preceding formulas, the oxidation of NADH has a standard potential of +0.32 V; therefore, the standard free‐energy change associated with the transfer of electrons from NADH to oxygen to make water is:
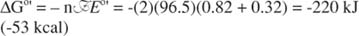
Compare this with the standard free‐energy change of making an ATP from ADP and inorganic phosphate: 31 kJ mol −1 (7.4 kcal mol −1). The purpose of the respiratory chain is to harness this large free‐energy change to efficiently synthesize ATP. Three ATP molecules are made by the respiratory chain during the transfer of electrons from NADH to O 2; this corresponds to an efficiency of about 40%. This efficiency is about the same as that of, for example, a diesel engine. Because over 90% of the adenosine nucleotides in the cell are normally fully converted into ATP, the concentration of reactants is lower than standard, and the concentrations of product (ATP) are higher than in the standard state. Thus, cells are able to convert electrical potential into chemical energy at high (although not 100%) efficiency.