Cells that are actively growing need an adequate supply of nucleotides to support RNA and DNA synthesis, and this reaction meets that need.
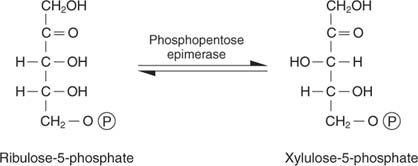
Alternatively, ribulose‐5‐phosphate can be converted into another 5‐carbon sugar by epimerization (change of one stereoisomer into another) into another pentose, xylulose‐5‐phosphate. This reaction is at equilibrium in the cell:
Converting pentoses to sugars
The pentoses are converted into 6‐ and 3‐carbon sugars. This reaction scheme appears complicated, and it is. The way to decipher it is to remember two key concepts:
- Either 3‐carbon units (one reaction) or 2‐carbon units (two reactions) are transferred between acceptor and donor molecules. The enzyme responsible for the 3‐carbon transfers is called transaldolase, and the enzyme that is responsible for the transfer of 2‐carbon units is called transketolase.
- The number of carbons involved in the reactions add up to either ten (two reactions) or nine (one reaction).
The first reaction has the shorthand notation:

which stands for the reaction of ribulose‐5‐phosphate and xylulose‐5‐phosphate with transketolase (2‐carbon transfer):

As shown in Figure 2, the 7‐carbon sugar, sedoheptulose‐7‐ phosphate, and the 3‐carbon sugar, glyceraldehyde‐3‐phosphate, react again, in a reaction catalyzed by transaldolase (3‐carbon transfer):

Figure 2
The overall conversion, then, is the conversion of two pentoses into a tetrose (4‐carbon) molecule and a hexose. Fructose‐6‐phosphate, the hexose, is a glycolytic intermediate and can enter that pathway at this stage. As shown in Figure 3, the 4‐carbon sugar, erythrose‐4‐phosphate, reacts with a molecule of xylulose‐5‐phosphate, catalyzed by transketolase (2‐carbon transfer):

The overall reaction scheme of the pentose phosphate pathway is:


Figure 3
In the sugar interconversion phase, three molecules of ribulose‐ 5‐phosphate have thus been converted to two molecules of fructose‐ 6‐phosphate and one molecule of glyceraldehyde‐3‐phosphate. These molecules are glycolytic intermediates and can be converted back into glucose, which can, of course, be used for the synthesis of glycogen.
Catabolism of other carbohydrates
The catabolism of other carbohydrates involves their conversion into glycolytic intermediates. Humans encounter a variety of
disaccharides (two‐sugar compounds) in their diet. Glycerol is a product of fat (triglyceride) digestion.
Lactose (glucosyl‐galactose) is predominant in milk, the primary nutrient for mammalian infants.
Mannose (glucosyl‐glucose) and
sucrose (glucosyl‐fructose) are ingested from cereals and sugars. The first step in their utilization is their conversion to monosaccharides by specific hydrolytic enzymes known as glucosidases. A deficiency in these enzymes can cause a variety of gastrointestinal complaints as the unhydrolyzed disaccharides are poorly absorbed in the small intestine. If not absorbed, the carbohydrates pass into the small intestine, where they feed the bacteria there. The bacteria metabolize the sugars, causing diarrhea and flatulence.
Lactase, the enzyme responsible for lactose hydrolysis, is not synthesized after weaning by most humans. If these individuals consume dairy products, they show symptoms of lactose intolerance. Addition of purified lactase to milk predigests the lactose, often preventing the symptoms.
Before galactose can be metabolized by the glycolytic pathway, it must be converted into glucose‐6‐phosphate. The first step in the process is the phosphorylation of galactose into galactose‐1‐phosphate by galactokinase.
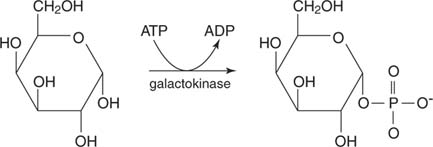
Then galactose‐1‐phosphate is transferred to a UMP nucleotide by reaction with the sugar nucleotide, Uridine diphosphate glucose (UDP‐glucose). This reaction liberates glucose‐1‐phosphate, which is converted into glucose‐6‐phosphate by phosphoglucomutase (see Figure 4). (This enzyme is also important in the breakdown of glycogen.)

Figure 4
UDP‐glucose is initially formed by reaction of glucose‐1‐phosphate with UTP and the release of inorganic pyrophosphate (see Figure 5).

Figure 5
Finally, UDP‐galactose is epimerized to UDP‐glucose by the action of UDP‐galactose epimerase (see Figure 6). This UDP‐glucose can be used in the galactosyltransferase reaction.

Figure 6
This elaborate scheme is probably due to the need to guard against the toxic buildup of galactose‐1‐phosphate. Humans who lack the enzymes required for galactose epimerization because they have a genetic deficiency of the enzyme suffer from mental retardation and cataracts. In microorganisms, expression of galactokinase in the absence of the epimerase and transferase inhibits cell growth.