Acid‐Base Reactions in Living Systems
Biochemists usually discuss acids and bases in terms of their ability to donate and accept protons; that is, they use the Brønsted definition of acids and bases. A few concepts from general chemistry are important to help organize your thoughts about biochemical acids and bases:
- A compound has two components — a conjugate acid and a conjugate base. Thus, you can think of HCl as being composed of the proton‐donating acidic part (H +) and the proton‐accepting basic part (Cl −). Likewise, acetic acid is composed of H + and the conjugate base (H 3CCOO −).

- The stronger the acid, the weaker its conjugate base. Thus, HCl is a stronger acid than acetic acid, and acetate ion is a stronger base than chloride ion. That is, acetate is a better proton acceptor than is chloride ion.
- The strongest acid that can exist in appreciable concentration in a solution is the conjugate acid of the solvent. The strongest base that can exist in a solution is the conjugate base of the solvent. In water, the strongest base that exists is OH −. If a stronger base, such as NaOCH 3, is added to water, the methoxide ion rapidly removes protons from the solvent:

leaving the base OH ‐ as the strongest base in solution. (Don't try these reactions at home; they are highly exergonic!) The strongest acid that can exist in water in appreciable amounts is H 3O +, the conjugate acid of H 2O:

- Weak acids and bases — those less strong than H + or OH − — exist in equilibrium with water:

pK values and protonation
The strength of an acid or base is given by its Ka or Kb, respectively. Ka × Kb = 1014, the dissociation constant of water. Just as it is convenient to describe the concentration of H+ ions in solution as:
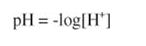
it is equally convenient to describe the Ka of an acid as its negative logarithm, so that:
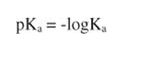
For example, acetic acid, which has a Ka = 1.74 × 10−5, has a pK = 4.76. Ammonia is more basic than water, with a pKa = 9.25, corresponding to its Ka = 5.6 × 10−10. If the pKa of a group is <7.0, it will donate a proton to water and a solution containing that compound will have a pH <7, that is, it will be basic. Conversely, if the pK of a compound is >7.0, that compound will accept a proton from water, and a solution containing that compound will have a pH >7, which puts it in the acidic range.
Solution pH
Many living organisms (there are many exceptions among the microbes) can exist only in a relatively narrow range of pH values. Thus, vegetables are often preserved by pickling them in vinegar, a dilute solution of acetic acid in water. The low pH of the solution prevents many bacteria and molds from growing on the food. Similarly, it is a cliche of movie Westerns that desert springs whose water is alkaline (basic) are decorated with the skulls of cattle who were unfortunate enough to drink from them. Finally, individuals with chest injuries who are unable to breathe efficiently develop a metabolic acidosis, as their blood pH drops below normal due to the impaired elimination of CO2 (a weak acid) from the lungs.
Microorganisms capable of living in acidic environments expend a large amount of energy to keep protons from accumulating inside their membranes. These examples show the importance of controlling the pH of biological systems: Biochemical reactions, and therefore life, can exist only in a narrow, near-neutral pH range.
All physiological pH control relies ultimately on the behavior of weak acids and bases as buffers. A buffer is a combination of a weak acid and its salt or a weak base and its salt. The addition of an acid or a base to a buffered solution results in a lesser pH change than would occur if the acid were added to water alone.
This behavior is described quantitatively by the Henderson-Hesselbach equation,which can be derived from the definition of Ka:
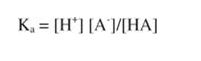
where HA is a weak acid — acetic acid, for example.
Taking the logarithm of each side of the equation:
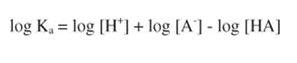
Remembering that:
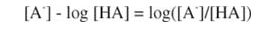
and multiplying through by (-1):
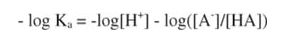
Rearranging, and remembering the definitions of pK and pH:
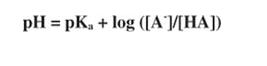
This equation allows you to predict the pH of a buffered solution from the values of the pKa and the amount of basic and acidic forms of the buffer. (For convenience, the subscript of the pK brackets to indicate concentration and charges are sometimes omitted, and the equation becomes pH = pK +log (A/HA).)
For example, calculate the pH of a solution containing 0.1 M acetic acid and 0.01 M sodium acetate
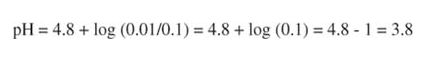
If the proportions of acid and salt are reversed, the pH would be 5.8. If they are equal, the ratio is:
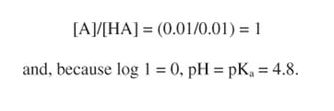
and, because log 1 = 0, pH = pKa = 4.8.
Buffer capacity
What would happen if 0.005 equivalents of a strong acid, for example, HCl, were added to each of the preceding three solutions? The strong acid would donate protons to the acetate ion present in each solution. This would change the ratio [A]/[HA], and consequently, the pH, in each case:
If the HCl is added to pure water, the pH of the solution changes from 7 to 1.3. Thus, in each case, the change in pH was less than would have been observed in the absence of buffer. The lowest pH change is seen in case 3. This illustrates a general rule: The amount of change in pH of a buffer system is lowest near the pKaof the conjugate acid. In other words, buffers have their highest capacity when the amounts of acidic and basic components are nearly equal. In practice, buffers are generally useful when the ratio A/HA is between 0.1 and 10; that is, at a pH within ± 1 pH unit of their pKs.
Metabolism occurs in cells at pH values near neutrality. For example, plasma must be maintained at a pH within half a pH unit of its normal value of 7.4. A number of mechanisms help accomplish this, including buffering by the mono- and di-basic forms of phosphate ion:
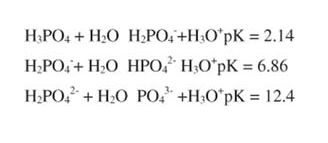
At physiological pH, the Henderson-Hesselbach equation shows that the second equilibrium is most important. Phosphoric acid and phosphate exist in vanishingly small quantities near neutrality.
When carbon dioxide is dissolved in water, it exists in equilibrium with the hydrated form:
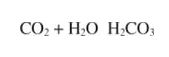
which is a weak acid. Carbonic acid, H2CO3, can donate two protons to a base:
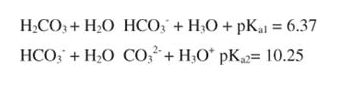
Metabolism releases CO2, which reduces the pH of the fluid around the cell and must be buffered for metabolism to continue. In animals, hemoglobin and other blood proteins play an important role in this buffering.