The photosynthetic scheme begins when the pigments of the light‐ harvesting complex absorb a photon of visible light. The absorption of light excites the pigment to a higher energy state. The pigments transfer their excitation energy to the reaction center chlorophyll molecule. Two types of reaction centers exist, generally called Photosystem I (PSI) and Photosystem II (PSII). Thus, two types of overall reactions make up photosynthesis—the light and dark reactions—while two more types of reactions compose the light reaction—those carried out by PSI and PSII. Absorption of a photon causes either type of chlorophyll to become more easily oxidized—that is, to give up an electron. The electron given up by either chlorophyll molecule flows through an electron transport chain, just as the electron given up by a mitochondrial cytochrome flows through the mitochondrial electron transport chain. Just as in mitochondrial electron transport, the flow of electrons leads to a proton gradient, which is used to synthesize ATP. Unlike mitochondrial electron transport, the terminal electron acceptor isn’t oxygen but rather NADP.
![]()
Another way of stating this relationship is that P680* has a more negative reduction potential than does P680. The following equation gives the free energy of the reduction reaction.

The conversion of the P680 to P680* means that the free energy of its accepting an electron becomes more positive (the reaction is unfavored). A molecule that has a more positive free energy of reduction has a more negative free energy of oxidation. In other words, the P680* molecule is more easily oxidized than is P680.
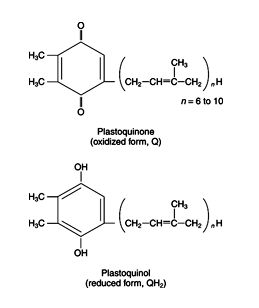
The electron from P680* is transferred to a series of plastiquinone (PQ) derivatives, leaving behind an oxidized P680 molecule, as shown in Figure . The reduction of plastoquinone is similar to that of Coenzyme Q in mitochondrial oxidation/reduction, in that PQ can accept either one or two electrons at a time. Plastiquinone molecules accept a proton (H+) from the stroma for each electron they accept. This leaves the stroma more basic than it was before, creating part of the gradient that will be used for ATP synthesis.

Figure 1
From the quinones, the electron is transferred to plastocyanin and then to cytochrome bf. The two H + ions (protons) left behind remain in the thylakoid lumen. As the electrons move down this electron transport chain, protons are pumped into the thylakoid lumen. Eventually the transported electron is given up to the oxidized P700 chlorophyll of Photosystem I.
This movement of an electron to PSI from P680* leaves P680 in a non‐excited, oxidized state. Oxidized P680 must be reduced to give up another electron. Hydrogen atoms derived from H 2O reduce it according to the reaction:

This oxidation transfers four electrons to the Manganese Center, a complex metalloprotein, which then donates the electrons through an intermediate to oxidized P680. The protons derived from water are transported into the thylakoid lumen. The protons pumped into the thylakoid lumen by PSII are used to make ATP through the action of coupling factor, in a mechanism similar to that of mitochondrial ATP synthesis.
Just as the absorption of a photon of light converts P680 to P680*, which is a better reductant, so too does the absorption of light convert the chlorophyll of PSI to a species that gives up an electron more easily. P700* donates an electron to a series of mobile quinones and then to a ferredoxin protein. The ferredoxin reduces NADP to NADPH. This provides reducing power for conversion of CO 2 to carbohydrate.

After P700* donates the electron to the quinones, oxidized P700 accepts an electron from the cytochrome bf, which is the acceptor of the electron from Photosystem II. The reduced P700 is now poised to absorb a photon’s energy and the cycle can start over.