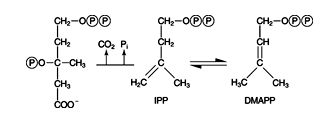
Mevalonate molecules are condensed to a 30‐carbon compound, squalene. The alcohol groups of mevalonate are first phosphorylated. Then they multiply phosphorylated mevalonate decarboxylates to make the two compounds isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP).
mevalonate → phosphomevalonate → pyrophosphomevalonate
This is a simple sequence of two phosphorylations using ATP as the donor: ![]()
![]()
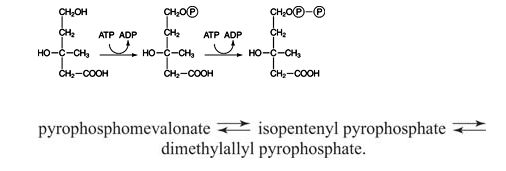
First, the other hydroxyl group of mevalonate accepts a phosphate from ATP. The resulting compound rearranges in an enzyme‐catalyzed reaction, eliminating both CO 2 and phosphate. The 5‐carbon compound that results, IPP, is rapidly isomerized with DMAPP. ![]()
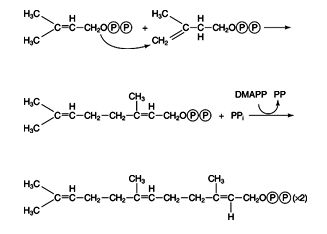
geranyl and farnesyl pyrophosphates → squalene
In plants and fungi, IPP and DMAPP are the precursors to many so‐called isoprenoid compounds, including natural rubber. In animals, they are mainly precursors to sterols, such as cholesterol. The first step is condensation of one of each to geranyl pyrophosphate, which then condenses with another molecule of IPP to make farnesyl pyrophosphate. Some important membrane‐bound proteins have a farnesyl group added on to them; however, the primary fate of farnesyl pyrophosphate is to accept a pair of electrons from NADPH and condense with another molecule of itself to release both pyrophosphate groups. ![]()
![]()
![]()
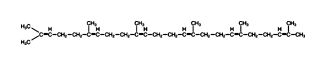
The resulting 30‐carbon compound is squalene; it folds into a structure that closely resembles the structure of the steroid rings, although the rings are not closed yet.
![]()