Nucleophilic Substitution Reactions: Mechanisms
Experimental data from nucleophilic substitution reactions on substrates that have optical activity (the ability to rotate plane‐polarized light) shows that two general mechanisms exist for these types of reactions. The first type is called an S N2 mechanism. This mechanism follows second‐order kinetics (the reaction rate depends on the concentrations of two reactants), and its intermediate contains both the substrate and the nucleophile and is therefore bimolecular. The terminology S N2 stands for “substitution nucleophilic bimolecular.”
The second type of mechanism is an S N1 mechanism. This mechanism follows first‐order kinetics (the reaction rate depends on the concentration of one reactant), and its intermediate contains only the substrate molecule and is therefore unimolecular. The terminology S N1 stands for “substitution nucleophilic unimolecular.”
The alkyl halide substrate contains a polarized carbon halogen bond. The S N2 mechanism begins when an electron pair of the nucleophile attacks the back lobe of the leaving group. Carbon in the resulting complex is trigonal bipyramidal in shape. With the loss of the leaving group, the carbon atom again assumes a pyramidal shape; however, its configuration is inverted. See Figure 1 below.

Figure 1
The S N2 mechanism can also be illustrated as shown in Figure 2.

Figure 2
Notice that in either picture, the intermediate shows both the nucleophile and the substrate. Also notice that the nucleophile must always attack from the side opposite the side that contains the leaving group. This occurs because the nucleophilic attack is always on the back lobe (antibonding orbital) of the carbon atom acting as the nucleus.
S N2 mechanisms always proceed via rearward attack of the nucleophile on the substrate. This process results in the inversion of the relative configuration, going from starting material to product. This inversion is often called the Walden inversion, and this mechanism is sometimes illustrated as shown in Figure 3.

Figure 3
S N2 reactions require a rearward attack on the carbon bonded to the leaving group. If a large number of groups are bonded to the same carbon that bears the leaving group, the nucleophile's attack should be hindered and the rate of the reaction slowed. This phenomenon is called steric hindrance. The larger and bulkier the group(s), the greater the steric hindrance and the slower the rate of reaction. Table 1 shows the effect of steric hindrance on the rate of reaction for a specific, unspecified nucleophile and leaving group. Different nucleophiles and leaving groups would result in different numbers but similar patterns of results.
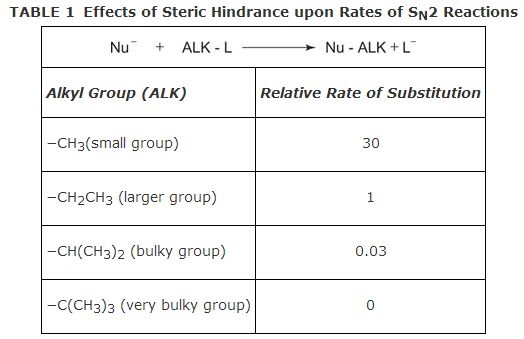
S N2 reactions give good yields on 1° (primary) alkyl halides, moderate yields on 2° (secondary) alkyl halides, and poor to no yields on 3° (tertiary) alkyl halides.
For protic solvents (solvents capable of forming hydrogen bonds in solution), an increase in the solvent's polarity results in a decrease in the rate of S N2 reactions. This decrease occurs because protic solvents solvate the nucleophile, thus lowering its ground state energy. Because the energy of the activated complex is a fixed value, the energy of activation becomes greater and, therefore, the rate of reaction decreases.
Polar aprotic solvents (solvents that cannot form hydrogen bonds in solution) do not solvate the nucleophile but rather surround the accompanying cation, thereby raising the ground state energy of the nucleophile. Because the energy of the activated complex is a fixed value, the energy of activation becomes less and, therefore, the rate of reaction increases.
Figure 4 illustrates the effect of solvent polarity on the energy of activation and, thus, the rate of reaction.

Figure 4
The smaller activation energy leads to the more rapid reaction.
SN1 mechanism
The second major type of nucleophilic substitution mechanism is the S N1 mechanism. This mechanism proceeds via two steps. The first step (the slow step) involves the breakdown of the alkyl halide into an alkyl carbocation and a leaving group anion. The second step (the fast step) involves the formation of a bond between the nucleophile and the alkyl carbocation.

Because the activated complex contains only one species—the alkyl carbocation—the substitution is considered unimolecular.
Carbocations contain sp 2 hybridized orbitals and thus have planar structures. S N1 mechanisms proceed via a carbocation intermediate, so a nucleophile attack is equally possible from either side of the plane. Therefore, a pure, optically active alkyl halide undergoing an S N1 substitution reaction will generate a racemic mixture as a product, as shown in Figure 5.

Figure 5
|
|
|
|